Abstract
Discontinuing unhealthy behaviors, such as overeating or drug use, depends upon an individual’s ability to overcome the influence of environmental reward cues. The strength of that influence, however, varies greatly depending upon the internal state of the body. Characterizing the relationship between interoceptive signaling and shifting drug cue valuation provides an opportunity for understanding the neural bases of how changing internal states alter reward processing more generally. A total of 17 cigarette smokers rated the pleasantness of cigarette pictures when they were nicotine sated or nicotine abstinent. On both occasions, smokers also underwent functional magnetic resonance imaging (fMRI) scanning while performing a visceral interoceptive attention task and a resting-state functional connectivity scan. Hemodynamic, physiological, and behavioral parameters were compared between sated and abstinent scans. The relationships between changes in these parameters across scan sessions were also examined. Smokers rated cigarette pictures as significantly more pleasant while nicotine abstinent than while nicotine sated. Comparing abstinent with sated scans, smokers also exhibited significantly decreased mid-insula, amygdala, and orbitofrontal activity while attending to interoceptive signals from the body. Change in interoceptive activity within the left mid-insula predicted the increase in smoker’s pleasantness ratings of cigarette cues. This increase in pleasantness ratings was also correlated with an increase in resting-state functional connectivity between the mid-insula and the ventral striatum and ventral pallidum. These findings support a model wherein interoceptive processing in the mid-insula of withdrawal signals from the body potentiates the motivational salience of reward cues through the recruitment of hedonic ‘hot spots’ within the brain’s reward circuitry.
Similar content being viewed by others
Introduction
It is part of the human condition that we struggle to discontinue unhealthy, but rewarding, behaviors. Be it dieting, smoking cessation, or any number of other unhealthy behaviors, humans share a common weakness: while sated, the prospect of quitting seems easy, but while starved, it feels nearly impossible. During these deprivation states, the reward cues in the environment exert a powerful hold, destroying resolve and provoking relapse. In short, the same stimulus—a cookie or a cigarette—can have very different incentive value depending on our body’s physiological state. It is for this reason that many theoretical accounts of addiction assert that the physiological and interoceptive symptoms of withdrawal serve to undermine cessation efforts by increasing the motivational salience of reward (ie, drug or food) cues (Droutman et al, 2015; Koob and Volkow, 2010; Naqvi and Bechara, 2010; Paulus et al, 2009). This process is thought to result from at least two sets of computations in the brain. First, the body’s current physiological state of withdrawal is compared with stored representations of the body at homeostasis. The aversive interoceptive state that results from this computation is sometimes referred to as a ‘body prediction error’ (Paulus et al, 2009). Second, the reward values of substances that can relieve this aversive interoceptive state, and consequently move the body back toward homeostasis, are adjusted upward. The increase in a stimulus’ reward value because of its potential to move the body’s physiological state toward homeostasis is often referred to as ‘positive alliesthesia’ (Cabanac, 1971). This positive alliesthesia for homeostatically relevant substances—such as food, drugs, and alcohol—thereby increases the likelihood of behaviors to procure those substances (Paulus et al, 2009). In short, the failure to reduce consumption of unhealthy rewards is grounded in how the bodily experience of withdrawal potentiates the value of reward stimuli and reward-related cues. Given the centrality of positive alliesthesia to addiction, it is important to delineate the specific brain processes that underlie the shift in reward cue valuation as a function of shifting interoceptive experience.
Many of the physiological signals indicating the state of the body, such as the somatic symptoms of drug withdrawal, reach the brain via the visceral afferent pathway that relays peripheral visceral signals through the vagus nerve to the nucleus of the solitary tract (nTS) in the medulla (Craig, 2002). Visceral afferent projections are then relayed through the pons and the thalamus to their primary cortical destination in granular–dysgranular regions of the posterior/mid-insula and frontoparietal operculum (Pritchard et al, 1986). In humans, the involvement of this region of the insula in interoception is demonstrated in neuroimaging studies of direct visceral stimulation (Cameron, 2002; Paulus et al, 2012; Wang et al, 2008), as well as interoceptive attention to visceral signals (Simmons et al, 2013a). The insula’s role in interoception and homeostatic signaling likely underlies its involvement in the aversive interoceptive state of craving. For example, altered insula activity has often been observed in neuroimaging studies of drug craving and drug cue reactivity (Naqvi and Bechara, 2009; Tang et al, 2012; Wang et al, 2007) as well as in hunger-related responses to food cues (Malik et al, 2008; Porubska et al, 2006; Simmons et al, 2013b). In rodent models, inactivation of interoceptive regions of the insula has been demonstrated to disrupt nicotine self-administration (Forget et al, 2010), and in humans, lesions of the insula are associated with the dramatic cessation of cigarette craving and the disruption of smoking addiction (Naqvi et al, 2007). The insula also has significant anatomical connectivity to the ventral striatum (Fudge et al, 2005), and this may explain its involvement in the hedonic component of taste perception (Small, 2010). This evidence suggests a strong link between the activity of the interoceptive insula, the subjective experience of craving, and the hedonic processing of sensory stimuli.
The insula’s role in addiction, however, is not simply to represent interoceptive withdrawal signals. The insula is a functionally heterogeneous region, as repeatedly demonstrated in human neuroimaging meta-analyses (Kurth et al, 2010) and experimental studies (Simmons et al, 2013a), and as such, the regions of the insula previously implicated in drug cue reactivity and craving may not be specifically involved in interoceptive awareness. Rather, their activation may reflect greater focal attention (Nelson et al, 2010) or stimulus salience (Menon and Uddin, 2010; Touroutoglou et al, 2012) attributed to drug-related cues, especially during craving states. Similarly, the heterogeneous functional organization of the insula makes it somewhat more difficult to interpret the neuropsychological evidence showing loss of craving after insula lesions (Naqvi et al, 2007). Does the loss of craving arise from diminished interoceptive awareness of physiological signals associated with nicotine withdrawal, or diminished stimulus salience and attention to smoking cues? Unfortunately, few neuroimaging studies have examined the relationship between drug craving and brain activity associated with interoception, particularly within the insular cortex.
Given the strong theoretical connection between the insula, interoceptive processing, craving, and the psychopathology of addiction (Droutman et al, 2015; Garavan, 2010; Naqvi and Bechara, 2010; Paulus et al, 2009), the mid/posterior insula would seem a likely candidate to be the critical mediator of positive alliesthesia, linking visceral interoceptive signals from the body to altered reward valuations. Nicotine addiction provides a model system for testing this hypothesis. Tobacco use is responsible for 480, 000 deaths in the United States every year and is the leading preventable cause of death in the country (Bauer et al, 2014). In nicotine-addicted individuals, nicotine withdrawal results in a host of aversive physiological changes in the viscera (eg, decreased heart rate and blood pressure, muscular tension, and gastrointestinal discomfort; Kenny and Markou, 2001; Morrell et al, 2008). We predict that the perception of these physiological changes should result in altered activity within the interoceptive insula, in proportion to the degree of nicotine withdrawal. This altered insula activity should in turn lead to a corresponding increase in the hedonic value for smoking-related stimuli, through the insula’s connectivity to classic mesolimbic reward brain regions. To evaluate these predictions we recruited cigarette smokers to perform a task in which they provided ratings of the expected pleasantness associated with smoking cigarettes depicted in photographs. They performed this task on two separate days, on which they were either nicotine sated or nicotine abstinent. Smokers also underwent functional magnetic resonance imaging (fMRI) scanning on both days, during which they performed a task requiring interoceptive attention to visceral sensations as well as a resting scan for the examination of resting-state functional connectivity.
Materials and methods
Participants
A total of 17 native English-speaking daily cigarette smokers (9 female; age: (mean (SD))=33 (9); range=22–51) participated in this study. Smokers ranged from low to high levels of nicotine dependence (Fagerstrom Test for Nicotine Dependence: mean (SD)=4 (2); range=1–8) and smoked 16 (9) cigarettes per day (range=10–45). Exclusion criteria included significant psychiatric and neurological history, current psychotropic medication use, and conditions that are contraindicated for magnetic resonance imaging. See the Supplementary Methods for more details on participant inclusion/exclusion criteria. All subjects received compensation for their participation and provided written informed consent as approved by Western Institutional Review Board.
Experimental Design
Participants were scanned on two separate days, at least 7 days apart. Before nicotine-abstinent sessions, participants were instructed to not smoke or use any nicotine product for at least 4 h. This duration of nicotine abstinence has been demonstrated as sufficient to induce the early physiological and psychological symptoms of nicotine withdrawal (Morrell et al, 2008). During nicotine-sated sessions, participants smoked as normal before arrival for the study, with their last cigarette occurring ∼5 min before the beginning of the brain scan. The order of nicotine-sated and nicotine-abstinent scans was counterbalanced across participants. Before scanning, participants completed the Questionnaire of Smoking Urges (QSU) to assess current craving. Immediately before both scan sessions, subjects’ levels of exhaled carbon monoxide (CO) were measured using a handheld CO meter in order to verify smoking status and quantify recent cigarette consumption.
On both scan days, participants received a series of structural MRI and fMRI scans (Supplementary Figure S1). While undergoing the fMRI scans, both groups of participants performed (1) an 8-min eye-open resting scan, (2) the Interoceptive Attention (IA) task, and (3) the Smoking Pleasantness (SP) task.
Interoceptive attention task
The IA task was composed of three 550 s fMRI scans, during which the participants performed two experimental conditions, the interoception condition and the exteroception condition (Supplementary Figure S2). During the interoception condition, the words ‘HEART,’ ‘STOMACH,’ and ‘BLADDER’ were individually presented on the screen for 10 s and subjects were instructed to focus their attention on interoceptive sensations from that organ. For example, upon seeing the word ‘HEART’, subjects focused on how intensely they could feel the sensation of their heart beating. Upon seeing the word ‘STOMACH’ or ‘BLADDER,’ subjects focused on the fullness of their stomach or bladder. During the exteroception control condition, the word ‘TARGET’ was presented in the middle of the screen and the word alternated from black to a lighter shade of gray every second. The subjects were instructed to focus their attention on the intensity of these color changes. Immediately after one-half of the trials of each condition, subjects were given a 5 s response period to make ratings, using an MR-compatible scroll-wheel, of the intensity of visceral sensations or the intensity of the color change of visual targets (with ‘1’ indicating little sensation/color change, ‘4’ indicating moderately intense sensation/color change, and ‘7’ indicating an extremely intense sensation/color change), during the preceding trial. Each condition trial was separated by a variable-duration interstimulus interval lasting between 2.5 and 22.5 s (mean interval=6.7 s), during which time subjects saw only a black fixation mark against a white background. By comparing internally directed with externally directed attention, this task utilizes the ‘attentional spotlight’ effect, the ability of attention focus to amplify the signal within primary cortical regions associated with a particular sensory modality, such as sight, sound, or touch (Brefczynski and DeYoe, 1999; Jancke et al, 1999; Johansen-Berg et al, 2000). This task has previously been used to identify interoceptive regions of the insula in healthy subjects (Avery et al, 2015; Simmons et al, 2013a) and to identify differences in interoceptive insula activity in clinical populations with major depressive disorder (Avery et al, 2014) and anorexia nervosa (Kerr et al, 2016). Immediately following each task scanning run, subjects reported current levels of cigarette craving (on a 1-to-9 scale) using the scroll wheel. See the Supplementary Methods for more IA task and stimulus presentation details.
Smoking pleasantness task
During the SP task (Supplementary Figure S2), subjects were presented with tobacco-related images selected from the Geneva Smoking Pictures database (Khazaal et al, 2012). The selected images consist of two separate types of smoking cues: Product pictures that depict various product-related cigarette cues such as cigarette packs, cartons, or discarded cigarette butts, and Smoking pictures that depict people in the act of smoking a cigarette. The selected images were all previously normed for valence, arousal, and dominance (Khazaal et al, 2012). During the presentation of the tobacco-related images, the subject rated on a 7-point scale ‘How pleasant would it be to smoke this cigarette right now?’ On the response scale, ‘1’ was depicted as ‘neutral’ and seven as ‘extremely pleasant.’ The pleasantness scale also included an ‘unpleasant’ option represented by the letter ‘X’ located below the number line. Subjects were instructed to select the ‘X’ if they believed it would be unpleasant to smoke the depicted cigarette.
Magnetic Resonance Imaging
Functional and structural MR images were collected using a General Electric Discovery MR750 (GE Healthcare, Milwaukee, WI) whole-body 3-Tesla MRI scanner. Pulse-oximetry recordings were used to calculate heart rate during functional scans. Functional imaging data were analyzed using the AFNI software package. The IA task data were analyzed at the subject level using a multiple linear regression model with regressors for each task condition plus regressors of noninterest (eg, subject motion parameters). See the Supplementary Methods for details on imaging parameters and preprocessing, subject-level statistical analysis, and heart rate analysis.
Statistical Analyses
Behavioral and physiological data
Paired t-tests were used to measure differences in the following measures between nicotine-sated and nicotine-abstinent scans: behavioral assessments, exhaled CO before scanning, visceral intensity and cigarette craving ratings (during IA task), and average heart rate during scanning. Smokers’ average pleasantness ratings during the SP task were also calculated for both task sessions, and the difference in average pleasantness rating (ΔSP; abstinent-sated) was computed for each subject. Pearson’s product-moment correlations were used to measure the relationships between changes in behavioral and physiological measures between sated and abstinent scans.
Imaging data
At the group level, the IA task data were analyzed using a repeated-measures paired t-test created using the whole-brain regression coefficients calculated at the subject level for the smokers’ nicotine-sated and nicotine-abstinent scan sessions. This test was designed to identify brain regions that exhibit a difference in the blood oxygenation level-dependent (BOLD) response to interoceptive vs exteroceptive attention (heart, stomach, and bladder interoception vs the exteroceptive attention control) between abstinent and sated scans. All statistical maps were cluster size corrected for multiple comparisons (see Supplementary Materials for details).
Next, a series of region-of-interest (ROI) analyses were performed to examine the relationship between abstinence-induced differences in interoceptive activity and pleasantness ratings of smoking pictures (ΔSP). Within each of the brain regions identified in the IA task contrast map detailed above (see Figure 1), the average regression coefficient for interoceptive attention (heart, stomach, and bladder interoception) from both scans was extracted, the difference between abstinent and sated scans was calculated (ΔIA), and Pearson’s product-moment correlation was used to examine the relationship between ΔIA and ΔSP. These ROI analyses were Bonferroni corrected for multiple comparisons. Subsequently, a mediation analysis was performed (Baron and Kenny, 1986) to determine whether ΔIA in left dorsal mid-insula (which was significantly related to ΔSP) mediated the relationship between ΔSP and the difference in exhaled carbon monoxide (ΔCO) (see Supplementary Materials for details).
Nicotine abstinence decreases interoception-evoked brain activity within interoceptive and appetitive neurocircuitry. Daily cigarette smokers exhibit decreased hemodynamic activity during interoceptive (vs exteroceptive) attention while in a nicotine-abstinent state, compared with that in a nicotine-sated state, in multiple brain regions involved in appetitive and affective processing. Significantly decreased IA task activation was observed within a network of limbic and paralimbic brain regions including the bilateral dorsal mid-insula, amygdala, and orbitofrontal cortex, regions that have been previously identified as playing a key role in the experience of drug craving.
Resting-state functional connectivity analysis
The left dorsal mid-insula ROI, the only region that exhibited a significant relationship between ΔIA and ΔSP, was used as a seed region for the analysis of resting-state functional connectivity. The average time course from this region during the resting-state task was used to examine differences in subjects’ resting-state functional connectivity between sated and abstinent scans, with the difference in pleasantness ratings (ΔSP) used as a covariate. This enabled the identification of regions of the brain whose difference in functional connectivity to the left dorsal mid-insula was predictive of the difference in inferred pleasantness of smoking stimuli. The resulting statistical map was cluster size corrected for multiple comparisons (see Supplementary Methods).
Results
Behavioral and Physiological Results
Behavioral and physiological correlates of nicotine craving
Before nicotine-abstinent scans, smokers abstained from smoking for an average (SD) of 7 (4) h. As expected, smokers’ exhaled CO levels were significantly lower when nicotine abstinent than when nicotine sated (ΔCO: t(16)=−8.4, p<0.001; Table 1) and self-reported cigarette craving, measured before scanning (QSU) and by craving rating scale (CRS) during scanning, was significantly higher when nicotine abstinent than when nicotine sated (p<0.001; Table 1). Consequently, smokers average pleasantness ratings for tobacco-related images during the SP task were also significantly greater while nicotine abstinent than while nicotine sated (ΔSP: t(16)=3.5, p<0.003; Table 1). The increase in cigarette cue pleasantness between scans was significantly predicted by a decrease in cigarette consumption (measured by exhaled CO: r (15)=−0.54; p<0.02; Supplementary Table S1) and an increase in cigarette craving (measured by CRS: r (15)=0.50, p<0.04; Supplementary Table S1). Between sated and abstinent scans, clinical measures of depression or anxiety did not change (p>0.28; Table 1). We did not observe any significant relationships between nicotine dependence, as measured by the Fagerstrom Test for Nicotine Dependence (FTND), and differences in exhaled CO, heart rate, cigarette craving, SP task ratings, or IA task activity (p>0.11; Supplementary Table S2). See Supplementary Materials for additional behavioral results.
Nicotine withdrawal was associated with altered physiological activity and interoceptive experience
Smokers’ heart rates during scanning were significantly greater during nicotine-sated than nicotine-abstinent scans (Abstinent-Sated: −5.5 BPM, t(16)=−5.4, p<0.001; Table 1 and Supplementary Figure S3). Conversely, smokers rated the intensity of their heartbeat sensations as higher during nicotine-abstinent scans (t(16)=2.1, p=0.05; Table 1 and Supplementary Figure S3). Stomach and bladder intensity ratings were also increased between sated and abstinent scans, but not significantly so (p>0.10; Table 1).
Altered interoceptive experience is positively related to changes in reward cue value
The change in the self-reported intensity of interoceptive sensations during the IA task was positively related to the change in pleasantness ratings during the SP task (r(15)=0.58, p<0.02; Supplementary Table S1).
Functional Neuroimaging Results
Nicotine abstinence alters interoception-related brain activity within interoceptive and appetitive neurocircuitry
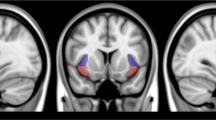
The effects of nicotine withdrawal on activity during the IA task were observed in a network of limbic and paralimbic brain regions including the bilateral mid-insula, bilateral amygdala, and left and right lateral orbitofrontal cortex (Figure 1 and Table 2). These regions all exhibited significantly decreased hemodynamic activity for interoceptive vs exteroceptive attention during nicotine-abstinent as compared with nicotine-sated scans. Decreased activity during nicotine abstinence was also observed in the left postcentral gyrus and left supplementary motor area (Table 2).
Decreased interoceptive mid-insula activity during nicotine abstinence is related to increased reward valuation for cigarette cues
Of all of the regions identified above (see Figure 1), only the left dorsal mid-insula exhibited a significant relationship between the difference in BOLD response to interoceptive attention (ΔIA) and the difference in pleasantness ratings for smoking pictures (ΔSP) between sated and abstinent scans (r(13)=−0.70, r2=0.49, p<0.004; Figure 2 and Supplementary Table S3).
Decreased interoceptive mid-insula activity during nicotine abstinence is related to positive alliesthesia for smoking pictures. Of all of the brain regions identified in the IA task contrast (Figure 1), only the left dorsal mid-insula exhibited a significant relationship between the difference in BOLD response to interoceptive attention (ΔIA) between sated and abstinent scans and the difference in pleasantness ratings for smoking pictures (ΔSP). Notably, both ΔSP and ΔIA were also significantly related to the difference in exhaled carbon monoxide between scans (ΔCO). Importantly, ΔIA in the left dorsal mid-insula partially mediates the relationship between ΔCO, a biomarker of cigarette consumption, and the change in pleasantness ratings for smoking pictures. This indicates that, the greater the reduction in recent cigarette use, the greater the decrease in interoceptive insula activity, and the greater the increase in the perceived pleasantness of smoking pictures.
Interoceptive mid-insula activity partially mediates the relationship between reduced cigarette consumption and increased reward value for cigarette cues
Notably, the ΔIA within the left dorsal mid-insula was significantly positively related to ΔCO (r(13)=0.56, p<0.03; Figure 2 and Supplementary Table S1). Because a significant relationship also existed between ΔCO and ΔSP (Figure 2 and Supplementary Table S3), a mediation analysis was conducted (Baron and Kenny, 1986) Values in bold font indicate significant differences between abstinent and sated scan sessions. to determine whether the relationship between these two variables was mediated by the change in interoceptive activity in the left dorsal mid-insula. The results of this analysis identified that ΔIA in the left dorsal mid-insula partially mediates the relationship between differences in exhaled CO and pleasantness ratings for smoking pictures (Figure 2 and Supplementary Table S4).
Increased mid-insula connectivity to reward neurocircuitry is related to increased reward value for cigarette cues
The resting-state time course from the left dorsal mid-insula ROI (Figure 1) was used to examine differences in subjects’ resting-state functional connectivity between sated and abstinent scans using the difference in SP task ratings as a covariate. Specifically, abstinence-induced increase in the pleasantness ratings for tobacco-related images was positively correlated to increased resting-state functional connectivity between the left dorsal mid-insula and a region of the left ventral striatum—which included the accumbens area—as well as a region within the right ventral pallidum (Figure 3 and Supplementary Table S5).
Increased interoceptive insula functional connectivity is related to positive alliesthesia for smoking pictures. The average time course from the left dorsal mid-insula ROI (Figure 1) was used to examine differences in subjects’ resting-state functional connectivity between sated and abstinent scans, using the change in average pleasantness ratings from the SP task as a covariate. Abstinence-induced increase in the pleasantness ratings of smoking images is significantly related to increased dorsal mid-insula connectivity to the left ventral striatum and the right ventral pallidum. This suggests that processing in the mid-insula of afferent withdrawal signals from the body amplifies the perceived hedonic value of smoking cues through increased connectivity to the subcortical brain regions most directly implicated in the processing of salience and reward.
Discussion
Positive alliesthesia, that is, an increase in the reward value of a sensory stimulus in response to changes in the internal milieu of an organism, has been conceived as an allostatic mechanism, increasing the incentive salience of environmental cues for stimuli that can return the body to homeostasis (Paulus et al, 2009). The insula’s involvement in subjective interoceptive awareness of the body (Craig, 2002), combined with evidence that insula lesions disrupt craving (Naqvi et al, 2007), suggests that it would be the primary cortical structure mediating the relationship between the perception of aversive interoceptive states and the potentiation of reward cues. Using nicotine craving in cigarette smokers as a model system for positive alliesthesia, this is precisely what we observed. During nicotine abstinence, smokers exhibited decreased hemodynamic activity during interoceptive attention to visceral signals within a network of regions involved in viscerosensation, arousal, and reward, including the bilateral orbitofrontal cortex, amygdala, and the mid-insula. Activation of each of these regions has been previously associated with exposure to both food and smoking cues (McClernon et al, 2005; Tang et al, 2012), an association that may reflect their more general role in the affective and motivational processing of sensory stimuli (Kenny, 2011; Koob and Volkow, 2010). However, only the change in interoceptive activity of the left dorsal mid-insula predicted the increase in hedonic ratings for cigarette cues during nicotine withdrawal. The increase in reward valuation for cigarette cues was also predicted by an increase in the left mid-insula’s functional connectivity to the ventral striatum and ventral pallidum, two primary hedonic hot spots within the brain’s reward circuitry (Haber and Knutson, 2010). These findings heavily support the dorsal mid-insula’s role as a critical neural mediator of positive alliesthesia.
One account of the present findings comes from the recently proposed Embodied Predictive Interoception Coding (EPIC) model that delineates the role of the mid-insula in interoception (Barrett and Simmons, 2015). Specifically, the granular/dysgranular region of the mid-insula compares ascending viscerosensory signals from the body with interoceptive predictions about the body’s state, generated by agranular visceromotor cortices (Barrett and Simmons, 2015). The differences between these signals, computed as prediction errors, are returned to agranular visceromotor cortices where they are used to update the computation of subsequent interoceptive predictions. According to the EPIC model, drug craving can be interpreted as a negative prediction error arising from the deviation between the brain’s predictions about the body’s prior physiological state, given typical homeostatic drug levels, and its current physiological (withdrawal) state. As in the generation of negative reward prediction errors in the striatum (Schultz et al, 1997), this negative interoceptive prediction error might also be characterized by a decrease, rather than an increase, in neural firing. Consistent with this theory, during nicotine withdrawal, cigarette smokers exhibited significantly decreased mid-insula activity during interoceptive attention, coupled with significant changes in physiological and behavioral measures of interoceptive experience. Furthermore, the magnitude of this decrease in insula activity was significantly related to the magnitude of cigarette abstinence, measured by decrease in exhaled carbon monoxide, an objective clinical biomarker of cigarette consumption. These results support the mid-insula’s potential role in generating these negative interoceptive prediction errors, as the greater the degree of nicotine abstinence in these individuals, the greater the disturbance of interoceptive mid-insula activity. Similar results have been observed using this same task in patients with depression (Avery et al, 2014) and anorexia (Kerr et al, 2016) who exhibit chronic somatic and visceral complaints, but paradoxically exhibit less interoception-evoked activity in these brain regions than healthy subjects (Avery et al, 2014).
During each study visit, smokers were also asked to rate the expected pleasantness of smoking cigarettes depicted in a series of photographs. These pleasantness inferences, which are themselves predictions of the interoceptive consequences of smoking the depicted cigarettes, were significantly greater during nicotine-abstinent as compared with nicotine-sated sessions. The magnitude of this increase in hedonic ratings was significantly related to the magnitude of cigarette abstinence (measured by change in exhaled carbon monoxide) as well as the magnitude of decreased left mid-insula activity during interoception. Importantly, the relationship between reduced cigarette consumption and increased reward value for cigarette cues was partially mediated by this change in interoceptive mid-insula activity. Moreover, those individuals who exhibited the greatest increase in hedonic ratings for smoking cues also exhibited the greatest increase in resting-state functional connectivity between the dorsal mid-insula and the ventral striatum and ventral pallidum, the regions of the brain’s reward circuitry most heavily implicated in hedonic experience (Haber and Knutson, 2010). Taken together, these results suggest that abstinence-induced alliesthesia for reward cues results from the computation of a negative prediction error within the mid-insula in response to afferent signals of withdrawal, and the provision of these signals to striatopallidal reward circuitry. The ventral striatum and ventral pallidum then use this information to mark stimuli capable of returning the body to homeostasis with greater motivational salience (Wyvell and Berridge, 2000) and hedonic reward (Berridge and Kringelbach, 2015; Smith et al, 2009).
The activity of the dorsal mid-insula has been associated with cue reactivity for alcohol and multiple drugs of abuse (Naqvi and Bechara, 2009), as well as homeostatically sensitive responses to food pictures (Simmons et al, 2013b), suggesting that the mid-insula’s role as a neural mediator of positive alliesthesia extends beyond nicotine addiction specifically. Consequently, the mid-insula may serve a more general function of regulating the incentive salience attributed to all homeostatically relevant stimuli (ie, food, water, addictive drugs) by connecting interoceptive signaling of changes in the internal milieu to the brain’s intrinsic reward circuitry. The necessity of this pathway for intact drug-seeking behavior is supported by translational research in rodents, where temporary inactivation of the interoceptive insula disrupts drug-induced place preference (Contreras et al, 2007) and drug self-administration (Forget et al, 2010), and by clinical research in human patients identifying that insula lesions disrupt addiction (Naqvi et al, 2007). Similarly, in psychiatric disorders such as anorexia and major depression, abnormal interoceptive insula activity is associated with abnormal feeding behavior (Avery et al, 2014; Kerr et al, 2016; Simmons et al, 2016). Taken together, the identification of this relationship between craving and interoceptive activity in the insula may serve to identify specific neural targets for interventions to help humans discontinue unhealthy, but rewarding, behaviors (see Supplementary Materials for limitations and additional discussion).
Funding and disclosure
The authors declare no conflict of interest.
References
Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK (2014). Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry 76: 258–266.
Avery JA, Kerr KL, Ingeholm JE, Burrows K, Bodurka J, Simmons WK (2015). A common gustatory and interoceptive representation in the human mid-insula. Hum Brain Mapp 36: 2996–3006.
Baron RM, Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51: 1173–1182.
Barrett LF, Simmons WK (2015). Interoceptive predictions in the brain. Nat Rev Neurosci 16: 419–429.
Bauer UE, Briss PA, Goodman RA, Bowman BA (2014). Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet 384: 45–52.
Berridge KC, Kringelbach ML (2015). Pleasure systems in the brain. Neuron 86: 646–664.
Brefczynski JA, DeYoe EA (1999). A physiological correlate of the 'spotlight' of visual attention. Nat Neurosci 2: 370–374.
Cabanac M (1971). Physiological role of pleasure. Science 173: 1103–1107.
Cameron OG (2002). Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosom Med 64: 851–861.
Contreras M, Ceric F, Torrealba F (2007). Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318: 655–658.
Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 33: 655–666.
Droutman V, Read SJ, Bechara A (2015). Revisiting the role of the insula in addiction. Trends Cogn Sci 19: 414–420.
Forget B, Pushparaj A, Le Foll B (2010). Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry 68: 265–271.
Fudge JL, Breitbart MA, Danish M, Pannoni V (2005). Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol 490: 101–118.
Garavan H (2010). Insula and drug cravings. Brain Struct Funct 214: 593–601.
Haber SN, Knutson B (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26.
Jancke L, Mirzazade S, Shah NJ (1999). Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett 266: 125–128.
Johansen-Berg H, Christensen V, Woolrich M, Matthews PM (2000). Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport 11: 1237–1241.
Kenny PJ (2011). Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 12: 638–651.
Kenny PJ, Markou A (2001). Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70: 531–549.
Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK (2016). Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology 41: 521–528.
Khazaal Y, Zullino D, Billieux J (2012). The Geneva Smoking Pictures: development and preliminary validation. Eur Addict Res 18: 103–109.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010). A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 214: 519–534.
Malik S, McGlone F, Bedrossian D, Dagher A (2008). Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7: 400–409.
McClernon FJ, Hiott FB, Huettel SA, Rose JE (2005). Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology 30: 1940–1947.
Menon V, Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667.
Morrell HE, Cohen LM, al'Absi M (2008). Physiological and psychological symptoms and predictors in early nicotine withdrawal. Pharmacol Biochem Behav 89: 272–278.
Naqvi NH, Bechara A (2009). The hidden island of addiction: the insula. Trends Neurosci 32: 56–67.
Naqvi NH, Bechara A (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct 214: 435–450.
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534.
Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010). Role of the anterior insula in task-level control and focal attention. Brain Struct Funct 214: 669–680.
Paulus MP, Flagan T, Simmons AN, Gillis K, Kotturi S, Thom N et al (2012). Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLoS One 7: e29394.
Paulus MP, Tapert SF, Schulteis G (2009). The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav 94: 1–7.
Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N (2006). Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage 32: 1273–1280.
Pritchard TC, Hamilton RB, Morse JR, Norgren R (1986). Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol 244: 213–228.
Schultz W, Dayan P, Montague PR (1997). A neural substrate of prediction and reward. Science 275: 1593–1599.
Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P (2013a). Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp 34: 2944–2958.
Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR et al (2016). Depression-related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry 173: 418–428.
Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ et al (2013b). Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci 16: 1551–1552.
Small DM (2010). Taste representation in the human insula. Brain Struct Funct 214: 551–561.
Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009). Ventral pallidum roles in reward and motivation. Behav Brain Res 196: 155–167.
Tang DW, Fellows LK, Small DM, Dagher A (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav 106: 317–324.
Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage 60: 1947–1958.
Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A et al (2008). Gastric distention activates satiety circuitry in the human brain. Neuroimage 39: 1824–1831.
Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP et al (2007). Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci 27: 14035–14040.
Wyvell CL, Berridge KC (2000). Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward ‘wanting’ without enhanced ‘liking’ or response reinforcement. J Neurosci 20: 8122–8130.
Acknowledgements
We thank Jennifer Dobson and Casey Mullins for assistance with subject assessment and recruitment, Jennifer Milsten for assistance with data management, as well as Justin Feinstein and Steve Green for helpful discussions. This research was supported by a grant from the Oklahoma Tobacco Research Center to WKS, a National Institute of Mental Health (K01MH096175-01) grant to WKS, and The William K Warren Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Avery, J., Burrows, K., Kerr, K. et al. How the Brain Wants What the Body Needs: The Neural Basis of Positive Alliesthesia. Neuropsychopharmacol 42, 822–830 (2017). https://doi.org/10.1038/npp.2016.128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.128
This article is cited by
-
An interoceptive basis for alcohol priming effects
Psychopharmacology (2021)