Abstract
Studies implicate opioid transmission in hedonic and metabolic control of feeding, although roles for specific endogenous opioid peptides have barely been addressed. Here, we studied palatable liquid consumption in proenkephalin knockout (PENK KO) and β-endorphin-deficient (BEND KO) mice, and how the body weight of these mice changed during consumption of an energy-dense highly palatable ‘cafeteria diet’. When given access to sucrose solution, PENK KOs exhibited fewer bouts of licking than wild types, even though the length of bouts was similar to that of wild types, a pattern that suggests diminished food motivation. Conversely, BEND KOs did not differ from wild types in the number of licking bouts, even though these bouts were shorter in length, suggesting that they experienced the sucrose as being less palatable. In addition, licking responses in BEND, but not PENK, KO mice were insensitive to shifts in sucrose concentration or hunger. PENK, but not BEND, KOs exhibited lower baseline body weights compared with wild types on chow diet and attenuated weight gain when fed cafeteria diet. Based on this and related findings, we suggest endogenous enkephalins primarily set a background motivational tone regulating feeding behavior, whereas β-endorphin underlies orosensory reward in high need states or when the stimulus is especially valuable. Overall, these studies emphasize complex interplays between endogenous opioid peptides targeting μ-receptors, such as enkephalins and endorphins, underlying the regulation of feeding and body weight that might explain the poor efficacy of drugs that generally target μ-opioid receptors in the long-term control of appetite and body weight.
Similar content being viewed by others
INTRODUCTION
Aberrant feeding, especially overeating, can lead to serious health problems, including obesity, diabetes, cardiovascular disease, and cancer (Guh et al, 2009). Opioid transmission has long been implicated in controlling feeding, energy storage, and body weight. However, most evidence implicating endogenous opioids is based on studies using general opiate antagonists such as naloxone and naltrexone (Gosnell and Levine, 2009; Yeomans and Gray, 2002), or genetic knockout of opioid receptors (Czyzyk et al, 2012; Farhang et al, 2010; Ostlund et al, 2013). In short, pharmacological studies are ill equipped to make definitive statements regarding the role of specific endogenous opioid peptides, such as β-endorphin vs enkephalins, in feeding. This is particularly pertinent considering that endogenous ligands of opioid receptors, such as β-endorphin and enkephalins, are promiscuous in their actions, potentially cross-activating μ (MOR) and δ (DOR) opioid receptors. Moreover, these endogenous ligands differ markedly in their distribution and expression levels (Akil et al, 1984).
A particularly well-established aspect of feeding modulated by opioid transmission is palatability (Yeomans et al, 2004). Indeed, the view that endogenous opioid activity, particularly β-endorphin signaling, underlies the pleasurable properties of food is long standing (see Nogueiras et al, 2012 for review). In general, opioids acting at MORs in limbic nuclei, including the nucleus accumbens and ventral pallidum appear to imbue food with a hedonic quality that ultimately promotes feeding, particularly the amount of time animals devote to feeding bouts before taking a break (Doyle et al, 1993; Giraudo et al, 1999).
Food stimuli and associated cues can attain incentive motivational properties that increase food consumption by promoting the initiation of food-seeking behavior. Recent studies suggest that endogenous opioids may not only mediate the hedonic properties of food, but also its motivational properties (Hayward et al, 2006; Mahler and Berridge, 2009; Wassum et al, 2011). Again, the largely pharmacological methods employed to date are unable to shed light on the identity of the endogenous opioids involved. The hedonic and motivational properties of food are likely to be very closely related under normal conditions, but may become dissociated in pathological states such as addiction or under specifically engineered experimental conditions (Berridge, 2009; Nogueiras et al, 2012; Pecina and Smith, 2010).
This study aimed to distinguish the role of the two major classes of endogenous opioids acting at MORs and DORs, namely enkephalins and β-endorphin, in the hedonic and motivational aspects of feeding behavior, as well as in body weight regulation. Microstructural analysis of licking behavior, commonly used to parse hedonic and motivational aspects of feeding (D'Aquila, 2010; Davis and Smith, 1988; Frisina and Sclafani, 2002; Higgs and Cooper, 1998; for review see Dwyer, 2012), was conducted in proenkephalin knockout (PENK KO) and β-endorphin-deficient (BEND KO) mice during consumption of sweet solutions, followed by measurement of body weight gain during long-term access to a ‘cafeteria diet’ (Sampey et al, 2011).
MATERIALS AND METHODS
Animal Subjects and Housing
Four groups of age-matched mice (average age of 152 days at start of experiment) were studied concurrently: PENK KOs (7 male and 6 female, König et al, 1996) and wild types derived from the same colony (9 male and 8 female), and BEND KOs (4 male and 4 female, Rubinstein et al, 1996) and wild types derived from the same colony (4 male and 4 female), produced by heterozygous–heterozygous matings. Mice were group housed in cages of mixed genotypes. Food and water were provided ad libitum (except as noted below) in a climate-controlled colony room (22 °C). All testing took place during the light cycle of a 12-h light/dark schedule (lights on 0600–1800 h). Experimental protocols were approved by the UCLA Institutional Animal Care and Use Committee and were performed in accord with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Apparatus
Licking microstructure was recorded in 30-min sessions in a darkened square chambered (10 × 10 cm) lickometer (see Supplementary Materials for further details). Digitized recordings of total number of spout licks, the average length of individual bouts of licking, and the total number of licking bouts were recorded.
Experimental Protocols
Training and rebaseline days in lickometer
Training and testing occurred in blocks of 5 days, with 2 days between blocks. On all training and testing days, mice were placed in the lickometer for 30 min with continuous and free access to sucrose solution. Mice were trained for two 5-day blocks with 10% sucrose, each daily exposure following 4 h of food deprivation, as previously described (Ostlund et al, 2013). On the first day of all 5-day testing blocks, mice were given a single test session using default training conditions (10% sucrose, 4 h food deprivation) to reestablish baseline responding. Concentration or food deprivation effects on licking behavior were tested on the 4 days following rebaseline day 1 (days 2–5 of the 5-day testing block). Analysis of both training and rebaseline days is presented in Supplementary Material.
Licking behavior in response to manipulations of sucrose concentration
Across two 5-day blocks, mice were given four tests with 2% and four tests with 20% sucrose, counterbalanced for order, all under 4 h food deprivation conditions.
Licking behavior in response to manipulations of food deprivation
Across two 5-day blocks of testing, mice were given four tests under 0 h and four tests under 18 h of food deprivation, counterbalanced for order, all with 10% sucrose concentration.
Licking behavior in response to manipulations of sucralose concentration
Sucralose is a noncaloric sweetener, and can be used to study consummatory behavior supported by hedonic processes in the absence of homeostatic metabolic influences (Bachmanov et al, 2001; Frank et al, 2008; Ostlund et al, 2013). Across one 5-day block of testing, mice were tested twice with a low (0.0003%) and twice with a high (0.003%) concentration of sucralose, counterbalanced for order, all under 4 h food deprivation.
Measurement of body weight and feeding behavior during access to cafeteria diet
All mice were weighed once weekly during the palatability testing described above. At 20 days after sucralose testing, mice were given 24 h access to an energy-dense highly palatable ‘cafeteria diet’ (CD) for 29 consecutive days in their home cages. The CD consisted of a mixture of standard lab chow and three to four randomly selected highly palatable ‘snacks’ available ad libitum (see Supplementary Materials). Each mixture was presented for 2–4 days. During CD access, animals were weighed daily. Individual ‘snapshot’ measures of CD consumption were made at two time points, providing a general measure of food intake (additional details provided in Supplementary Materials).
RESULTS
Palatability Responses
Licking behavior in response to manipulations of sucrose concentration
Across PENK KO mice and their respective wild types, a significant main effect of sucrose concentration was found for total licks (F(1, 26)=97.12, p<0.001, Figure 1a), bouts of licking (F(1, 26)=12.72, p<0.01, Figure 1b), and mean bout length (F(1, 26)=51.90, p<0.01, Figure 1c), such that all licking measures were higher for 20% than 2% sucrose, as expected. More importantly, significant main effects of genotype were apparent for total licks (F(1, 26)=12.48, p<0.01, Figure 1a) and bouts of licking (F(1, 26)=9.11, p<0.01, Figure 1b), but not mean bout length (Figure 1c), such that PENK KOs exhibited fewer total licks and licking bouts across the two sucrose concentrations. This general decrease in bout number may reflect a role of endogenous enkephalins in supporting incentive motivational aspects of feeding behavior (see Supplementary Materials for further discussion, D'Aquila, 2010; Davis and Smith, 1988; Higgs and Cooper, 1998). Interestingly, although there was no main genotype effect on bout length, there was a significant interaction between sucrose concentration and genotype for this measure (F(1, 26)=11.87, p<0.01, Figure 1c) because of PENK KOs exhibiting shorter licking bout lengths at 2% sucrose, but longer bouts at 20% sucrose. This interaction aside, the general similarity between PENK KO and wild-type mice in bout length, together with appropriate upregulation with increased sucrose concentration, suggests endogenous enkephalins play little role in palatability processing.
Licking measures for a 2 and 20% sucrose solution, following 4 h food deprivation, in PENK (a–c) or BEND (d–f) KOs and their respective wild-type mice. (a, d) Total licks during 30 min sessions for PENK KO (n=13), BEND KO (n=8), and their respective wild types (n=17 and n=8, respectively). (b, e) Total number of licking bouts during the session. (c, f) Mean length of licking bouts. Data were averaged across 4 test days for each animal and are presented as mean±SEM. Horizontal and vertical bars with asterisks (*) indicate statistically significant (p<0.05) main effects of sucrose concentration and genotype, respectively. Pound sign (#) indicates significant (p<0.05) day x genotype interaction.
Across BEND KO mice and their respective wild types, a significant main effect of sucrose concentration was found for total licks (F(1, 12)=53.93, p<0.001, Figure 1d) and mean bout length (F(1, 12)=37.42, p<0.001, Figure 1f); however, no effect was found for number of licking bouts (Figure 1e). More importantly, significant main effects were observed for genotype on mean bout length (F(1, 12)=8.57, p<0.05, Figure 1f), but not total licks (Figure 1d) or total bouts of licking (Figure 1e), with BEND KOs exhibiting shorter mean bout lengths than wild types. A significant two-way interaction was also found between sucrose concentration and genotype for this measure (F(1, 26)=10.40, p<0.001, Figure 1f). The post hoc comparisons at each concentration revealed a significant effect of genotype at 20% sucrose (t(14)=3.46, p<0.01), but not 2% (t(14)=1.68, p=NS), such that BEND KO had shorter bout lengths for 20% but not 2% (Figure 1f). That BEND KO mice exhibited deficits in the length of licking bouts but not total bouts suggests endogenous β-endorphin signaling is specifically involved in feeding driven by palatability, as opposed to incentive motivation, an action that appears to manifest at higher sucrose concentrations.
Licking Behavior in Response to Food Deprivation
Across PENK KO mice and their respective wild types, a significant main effect of food deprivation was found for total licks (F(1, 26)=156.39, p<0.001, Figure 2a), bouts of licking (F(1, 26)=225.23, p<0.001, Figure 2b), and mean bout length (F(1, 26)=26.49, p<0.001, Figure 2c), such that all licking measures were higher with 18 h food deprivation compared with 0 h food deprivation, as expected. Significant main effects were observed for genotype on total licks (F(1, 26)=11.25, p<0.01, Figure 2a) and bouts of licking (F(1, 26)=8.38, p<0.01, Figure 2b), but not bout length (Figure 2c), such that PENK KOs exhibited fewer total licks and licking bouts compared with wild types. Similar to that seen with manipulations of sucrose concentration, the decrease in total bouts, but not bout length, in PENK KO mice suggests endogenous enkephalin signaling is preferentially involved in incentive motivational aspects of feeding, rather than palatability, and is independent of food deprivation state.
Licking measures for a 10% sucrose solution, following 0 or 18 h food deprivation, in PENK (a–c) or BEND (d–f) KOs and their respective wild-type mice. (a, d) Total licks during 30 min sessions for PENK KO (n=13), BEND KO (n=8), and their respective wild types (n=17 and n=8, respectively). (b, e) Total number of licking bouts during the session. (c, f) Mean length of licking bouts. Data were averaged across 4 test days for each animal and are presented as mean±SEM. Horizontal and vertical bars with asterisks (*) indicate statistically significant (p<0.05) main effects of food deprivation and genotype, respectively. Pound sign (#) indicates significant (p<0.05) day x genotype interaction.
Across BEND KO mice and their respective wild types, a significant main effect of food deprivation was found for total licks (F(1, 12)=52.31, p<0.001, Figure 2d), total bouts (F(1, 12)=52.31, p<0.001, Figure 2e), and bout length (F(1, 12)=37.42, p<0.001, Figure 2f), such that an increase in all licking measures was apparent with 18 h food deprivation compared with 0 h food deprivation, as expected. Although no significant main effects of genotype were observed for any of the measures, significant food deprivation by genotype interactions were apparent for total licks (F(1, 12)=6.31, p<0.05, Figure 2d), bouts of licking (F(1, 12)=5.20, p<0.05, Figure 2e), and mean bout length (F(1, 12)=4.49, p=0.05, Figure 2f). The post hoc comparisons at each level of food deprivation revealed a significant effect of genotype on total licks (t(14)=2.49, p<0.05) and mean bout length (t(14)=2.26, p<0.05) but not total bouts of licking (t(14)=1.24, p=NS) with 18 h of food deprivation, but no effect of genotype was apparent for any of the licking measures with 0 h of food deprivation (ts(14)<1.08, ps=NS). Thus, the increased bout lengths at 18 h of food deprivation relative to 0 h of deprivation apparent in wild-type mice were attenuated in BEND KO mice (Figures 2d–f). Again, similar to manipulations of sucrose concentration, results seen with BEND KO mice suggests β-endorphin signaling preferentially supports feeding driven by palatability, as opposed to incentive motivation, and that this role dominates in states of high food deprivation (18 h).
Licking behavior in response to manipulations of sucralose concentration
Across PENK KO mice and their respective wild types, a significant main effect of sucralose concentration was found for total licks (F(1, 26)=27.73, p<0.001, Figure 3a), total bouts (F(1, 26)=16.02, p<0.001, Figure 3b), and mean bout length (F(1, 26)=20.62, p<0.001, Figure 3c), such that higher licking measures were observed with 0.03% compared with 0.003% sucralose. A significant main effect of genotype was apparent only for total licks (F(1, 26)=8.46, p<0.01, Figure 3a), with PENK KOs exhibiting fewer total licks across the two sucralose concentrations. The lack of a significant genotype effect on total bouts of licking for sucralose, compared with the significant reduction in total bouts of licking observed with sucrose, suggests enkephalin signaling in incentive motivation may be determined by nutritive properties, although the influence of floor effects to mask a more definitive role should be considered.
Licking measures for 0.003 and 0.03% sucralose solution, following 4 h food deprivation, in PENK (a–c) or BEND (d–f) KOs and their respective wild-type mice. (a, d) Total licks during a 30-min licking session for PENK KO (n=13), BEND KO (n=8), and their respective wild types (n=17 and n=8, respectively). (b, e) Total number of licking bouts. (c, f) Mean length of licking bouts. Data were averaged across 2 test days for each animal and are presented as mean±SEM. Horizontal and vertical bars with asterisks (*) indicate statistically significant (p<0.05) main effects of concentration and genotype, respectively.
Across BEND KO mice and their respective wild types, a significant main effect of sucralose concentration was found on test days for total licks (F(1, 12)=23.04, p<0.001, Figure 3d), bouts of licking (F(1, 12)=6.72, p<0.05, Figure 3e), and mean bout length (F(1, 12)=25.50, p<0.001, Figure 3f), such that an increase in all licking measures was observed with 0.03% compared with 0.003% sucralose. No significant effects of genotype were observed. These findings, compared with the observed significant decrease in mean bout length following manipulations of sucrose concentration, suggests the role of β-endorphin signaling in palatability is dependent, at least partially, on the nutritive properties of the food stimulus.
Body Weight Regulation
Body weights before long-term access to CD
Before exposure to the CD, PENK KO mice and their respective wild types displayed a significant main effect of time on body weights (F(6, 156)=8.70, p<0.001, Figure 4a) because of a slight increase in body weights in both PENK KO and wild-type mice over the 7-week measurement period (paired t-tests between week 1 and 7, t(29)=-2.80, p<0.01). A significant main effect of genotype (F(1, 26)=11.23, p<0.01) was also observed, such that PENK KOs weighed, on average, 10% less than wild types (Figure 4a). No significant differences in body weights were observed between BEND KOs and their respective wild types; however, contrary to that seen with PENK KOs, BEND KOs, on average, weighed 6% more than their respective wild types (Figure 4d).
Body weight before (a, d), during (b, e), and after (c, f) exposure to cafeteria diet in PENK (a–c) or BEND (d–f) KOs and their respective wild-type mice. (a, d) Weekly body weights for PENK KO (n=13), BEND KO (n=8), and wild-type mice (n=17 and n=8, respectively), during the 7 weeks of palatability testing preceding access to cafeteria diet. (b, e) Daily body weight during access to cafeteria diet, normalized to last standard chow access day (BL). (c, f) Body weights after access to cafeteria diet, normalized to last cafeteria diet access day (BL). Data are presented as mean±SEM. Horizontal and vertical bars with asterisks (*) indicate statistically significant (p<0.05) main effects of day and genotype, respectively. Pound sign (#) indicates significant (p<0.05) day x genotype interaction. Insets (b–f) show body weight in grams on baseline day—in these cases, horizontal bar with asterisks indicate statistically significant genotype effect.
Body weight gain and feeding behavior during access to CD
On baseline day, before CD exposure, average body weights for PENK KO mice were 12% lower than that of their respective wild types (t(28)=2.62, p<0.05, Figure 4b, inset). Furthermore, in PENK KO mice, there was a significant main effect of day on normalized body weight change (F(29, 754)=33.05, p<0.001, Figure 4b). A significant main effect of genotype was also observed (F(1, 26)=9.42, p<0.01), with wild-type mice showing greater relative increases in body weight than PENK KOs (Figure 4b). A significant interaction was also observed between day and genotype (F(29, 754)=5.58, p<0.001).
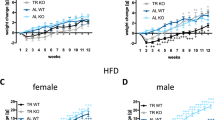
In the BEND KO study, no differences in absolute body weights were observed on baseline day (Figure 4e, inset). A significant main effect of day was apparent on normalized body weight change from baseline (F(29, 348)=25.46, p<0.001, Figure 4e), reflecting BEND KO mice and wild types both gaining weight during CD access. There was no significant main effect of genotype and no genotype × day interaction. However, BEND KO mice tended to gain more weight than wild types during the first 2 weeks of CD access, although this trend was not statistically significant (Figure 4e). Interestingly, when comparing diet-induced weight gain in BEND KOs with wild types, others have reported a more prominent and persistent obesity phenotype in male relative to female BEND KO mice (Appleyard et al, 2003). When comparing males and females separately in our study, both males and females showed significant weight gain over time across genotypes, that is, there was a significant main effect of day (Fs(29, 174)>12.05, ps<0.001). Although both male and female BEND KOs showed trends for greater body weight gain over time relative to wild types, no main effect of genotype was observed in either males or females (Figure 5). However, females, but not males, showed a significant genotype by day interaction (F(29, 174)=1.91, p<0.01), such that female BEND KOs gained more weight over time than wild types during initial CD access (CD days 1–24) and gained less weight toward the end of CD access (CD days 25–29). Male BEND KOs, on the other hand, trended toward greater weight gain across all CD diet days (except day 1) relative to wild types. Together with the results observed in the PENK study, these findings suggest that enkephalin signaling, as well as β-endorphin signaling, modulate body weight gain on CD.
Body weight during exposure to cafeteria diet in male and female BEND KOs and their respective wild-type mice. (a) Daily body weight gain for male BEND KOs and wild types during access to cafeteria diet, normalized to last standard chow access day (male wild type n=4, male BEND KO n=4). (b) Daily body weight gain for female BEND KOs and wild types during access to cafeteria diet, normalized to last standard chow access day (female wild type n=4, female BEND KO n=4). Data are presented as mean±SEM. Horizontal bar with asterisks (*) indicates statistically significant (p<0.05) main effects of day. Pound sign (#) indicates significant (p<0.05) day x genotype interaction.
Neither PENK nor BEND KO mice differed from wild types in calories obtained (normalized for body weight) from standard chow, CD, fat specifically, or sugar specifically during either snapshot tests 1 or 2 (Fs(1, 12)<3.16, ps>0.1, see Supplementary Material). Similar energy intake suggests the observed effects of CD on body weight gain may be driven by long-term differences in calorie consumption or processes other than caloric intake (eg, energy absorption, storage, fat oxidation).
Body weight loss following cessation of CD
On baseline day (ie, the last day of CD exposure), average absolute body weights for PENK KO mice were 17% lower than that of their respective wild types (t(28)=3.01, p<0.01, Figure 4c, inset). Both PENK KO and wild-type mice lost weight following return to chow-only feeding (main effect of day (F(6, 156)=17.08, p<0.001) on normalized weight change from baseline; Figure 4c), but this was less pronounced in PENK KO mice (main effect of genotype (F(1, 26)=7.29, p<0.05). A significant interaction between day and genotype (F(6, 156)=7.72, p<0.001) was also observed for normalized weight change from the post-CD baseline. Comparing normalized body weight change on the last day of post-CD access to the post-CD baseline showed that whereas wild-type mice had lost a significant amount of weight 3 weeks after CD access (t(16)=5.05, p<0.001), PENK KOs had not (t(12)=0.95, p=NS).
For BEND KO mice and their respective wild types, no differences in absolute body weight were observed on baseline day (Figure 4f, inset). A significant main effect of day (F(6, 72)=7.06, p<0.001) on normalized weight change from baseline when returned to standard rodent chow was seen (Figure 4f). No significant genotype main effect or interaction was observed during the 3 weeks following CD access.
Sex-dependent effects
Although main effects and interactions of sex were observed on some measures, no qualitative differences in responses for any manipulation existed. With the exception of those described above and discussed below, quantitative differences between the sexes are described in Supplementary Materials.
DISCUSSION
The main findings of this study are summarized in Table 1. Given that the number of bouts of licking initiated are thought to reflect the incentive motivational properties of the stimulus, whereas the length of bouts reflects hedonic properties (see Supplementary Materials for further discussion, D'Aquila, 2010; Davis and Smith, 1988; Higgs and Cooper, 1998), our results suggest endogenous enkephalins contribute to the overall motivational properties of palatable stimuli, independent of their hedonic impact. This finding is interesting for several reasons. First, it supports the notion that the incentive motivational property of a stimulus is distinct and dissociable from its hedonic property, a view that prevails heavily in the literature (see Berridge et al, 2009 for review). Second, this finding reveals that only a subset of endogenous opioid peptides—enkephalins—underlie incentive motivation, despite them sharing target receptors with other endogenous opioid peptides such as β-endorphin.
In contrast to PENK KO mice, BEND KO mice displayed bout numbers comparable to wild types, with shorter licking bout lengths, but only when sucrose concentration or food deprivation state was high. These findings suggest that endogenous β-endorphin underlies hedonic experiences, but not motivation for those experiences. Such an interpretation is based on an assumption that bout length and bout number are relatively pure measures of hedonic impact and motivation, respectively. Some overlap is perhaps to be expected, however, as the hedonic impact of a stimulus will ultimately influence motivation for that stimulus (ie, we want what we like). Nevertheless, licking bout length has been considered more immediately sensitive to hedonic impact, whereas the number of licking bouts across a session is generally considered to reflect motivation based on a long-run average of previous experience weighted only slightly toward recent events (D'Aquila, 2010; Davis and Smith, 1988; Frisina and Sclafani, 2002; Higgs and Cooper, 1998). However, some caution in this interpretation is called for in light of reports that dopaminergic antagonist effects on licking microstructure include decreases in bout duration (D'Aquila, 2010; Galistu et al, 2011; Genn et al, 2003; Liao and Ko, 1995; Schneider et al, 1990; Smith, 2004; for review see Dwyer, 2012), despite evidence that the mesolimbic dopamine system is a key mediator of motivated but not hedonic behavior (for review see Berridge and Robinson, 1998). Such effects on bout length have been interpreted in motivational terms, arguing that dopamine plays a role in the use of hedonic experience to extend motivation for consumption of palatable stimuli (D'Aquila, 2010). The same motivational interpretation might be applied to the attenuated bout lengths exhibited by BEND KO mice. Although it is tempting to argue that the reduction in bout length in BEND KO mice should be accompanied by a reduction in bout number under such a scenario (which was not the case), as indeed several studies using DA antagonists report (D'Aquila, 2010; Galistu et al, 2011; Schneider et al, 1990; Smith, 2004), exceptions to this pattern of DA antagonist action (D'Aquila, 2010; Galistu et al, 2011; Genn et al, 2003) require that a degree of caution be exercised in concluding that the selective reduction in bout length observed in the BEND KOs reflects a purely hedonic function of this peptide.
The supposition that endogenous enkephalins promote incentive motivation toward a palatable stimulus, and endogenous endorphins support their hedonic properties in high caloric need states, may reflect fundamental differences in their neuroanatomical distribution. Enkephalins are found widely distributed throughout the brain in limbic (eg, nucleus accumbens, ventral pallidum, central amygdala) and brainstem (eg, parabrachial nucleus) sites, many of which have well-studied roles in both hedonic processing and incentive motivation. Research has focused on the role of MOR activation within these regions, primarily in hedonic mechanisms (eg, hedonic hotspots), with roles in modulation of incentive motivation being less well studied. However, Berridge and colleagues (DiFeliceantonio et al, 2012) have recently demonstrated increases in extracellular endogenous enkephalins in the dorsal striatum following initiation of feeding of a palatable candy stimulus. The authors went on to show increases in palatable food intake (but not hedonic responses) with MOR stimulation in this same region and suggested that activation of MORs in the striatum may impact food motivation by altering presynaptic corticostriatal glutamate release or dopamine projections from the striatum to the substantia nigra (Bakshi and Kelley, 1993; Fujiyama et al, 2011; Jiang and North, 1992; Mena et al, 2011).
In contrast, central endorphins have a far more restricted distribution, being sourced almost entirely in the arcuate nucleus of the hypothalamus and projecting to a very limited number of brain nuclei such as the nucleus accumbens (Appleyard et al, 2003; Le Merrer et al, 2009). Nonetheless, endorphin-containing neurons are well positioned to relay ongoing peripheral metabolic states to the central nervous system and decades of research have emphasized that the β-endorphin-synthesizing neurons of the arcuate (ie, proopiomelanocortin neurons) are critical sensors of peripheral metabolic state, being sensitive to circulating glucose, leptin, and insulin (see Millington, 2007 for review). Thus, β-endorphin neurons are well positioned to respond to changing peripheral metabolic states by modifying the hedonic properties of food.
Although pharmacological and genetic knockout research approaches have been frequently used to investigate the role of opioid neurotransmission in behaviors related to energy intake and weight gain (Bodnar et al, 1995; Giraudo et al, 1999; Shaw et al, 1991; Zhang et al, 1998; Zuberi et al, 2008), these methods have also shed some light on the role of opioids in energy storage and expenditure (Czyzyk et al, 2012; Statnick et al, 2003; Tabarin et al, 2005a). Such studies show that transmission through opioid receptors modulates metabolic processes such as fat oxidation, independent of food intake (Tabarin et al, 2005b); however, such approaches are poorly suited for identifying roles for specific endogenous opioid ligands (Murphy, 2015). In the current study, PENK KO mice gain less weight while apparently consuming equal calories as wild-type mice. Clearly, further studies are warranted to determine the role of endogenous enkephalin, and other opioid peptides, in all aspect of energy balance.
As with sucrose, PENK KO mice emitted significantly less total licks for sucralose, although no significant reductions in the number of bouts or bout length were observed. However, a tendency toward reduced number of bouts was evident and the lack of statistical significance may reflect a generally lower potency of sucralose, compared with sucrose, to elicit licking in general. BEND KO mice barely distinguished themselves from wild-type mice in any of the licking measures when licking for sucralose, even with increases in concentration, that contrasts with their licking behavior during sucrose testing. Given that sucralose is a nonnutritive sweetener, that BEND KO mice are unperturbed in their responses to sucralose lends further support to the notion that endogenous β-endorphin acts only to support licking directed at stimuli of high value.
Both enkephalins and β-endorphin are predicted to bind and activate MORs in vivo, and the current findings are in line with recent observations in MOR KO mice (Ostlund et al, 2013). That is, these mice appear to exhibit the combined characteristics of PENK and BEND KOs in that they show fewer bouts of sucrose licking across varying levels of sucrose concentration and food deprivation, which is similar to PENK KO mice, but also show significantly shorter bout lengths than wild types when sucrose concentration or food deprivation state is high, which is similar to BEND KOs. However, it is important to bear in mind that these same endogenous ligands activate DORs. Given that DORs are expressed in enkephalin containing areas, our findings support studies suggesting that DORs are involved in incentive motivation (Laurent et al, 2012; 2014).
Studies using specifically engineered high-energy diets, such as those containing high fat, have also found resistance to obesity in MOR KOs (Czyzyk et al, 2012; Zuberi et al, 2008) and an obesity phenotype in BEND KOs (Appleyard et al, 2003). Interestingly, data from our study show that PENK KO mice weighed significantly less before CD access compared with respective wild-type mice and gained weight at a statistically reduced rate during exposure to CD. As far as we are aware, ours is the first study showing that PENK KOs are resistant to diet-induced obesity. Our snapshot measures of food consumption show no differences in CD consumption between PENK KO and wild types, and suggest that the effect of PENK KO on CD-induced weight gain are not easily explained by difference in CD consumption, and may instead relate to metabolic differences in energy utilization. However, the possibility that our limited snapshot measures were not sensitive enough to capture long-term differences in caloric intake (>24 h) should not be dismissed.
In contrast to that seen with PENK KOs, BEND KO mice did not significantly differ from wild types before or during access to CD (although they did show a consistent trend to weigh more at baseline and gain more weight early during CD access) and showed no differences from wild types in our snapshot consumption measures. These results contrast somewhat with a recent study showing that BEND KO mice can display significant weight gain when compared with wild types, as well as hyperphagia, when fed regular chow (Appleyard et al, 2003). Interestingly, the observed weight gain in the Appleyard study was limited to male BEND KO mice, suggesting that the role of β-endorphin in body weight gain may be sex dependent. Therefore, we conducted comparisons of wild types and BEND KOs weight gain during CD access within male or female mice separately. Interestingly, the transient increase in weight gain observed in females BEND KOs, as well as the persistent increase in weight gain observed in males BEND KOs, is indeed similar to that observed in the Appleyard study. Although we did not observe a significant increase in male BEND KOs relative to wild types, it should be noted that the Appleyard study assessed body weight gain during access to a standard rodent chow diet, whereas we investigated weight gain during access to CD.
Studies using multiple approaches (eg, pharmacological, genetic, neurochemical) have given rise to the long standing view that endogenous opioid activity signaling underlies the pleasurable properties of food (see Berridge et al, 2010; Kelley et al, 2005; Nogueiras et al, 2012 for reviews). Previous studies demonstrate that opioids acting at MORs appear to imbue food with a hedonic quality that promotes feeding (Doyle et al, 1993; Giraudo et al, 1999). In addition, recent studies suggest that endogenous opioids, such as enkephalin, can impact the motivational properties of food (Hayward et al, 2006; Mahler and Berridge, 2009; Wassum et al, 2011). Overall, our study provides support for these findings using a novel licking microstructure approach, and elucidates a complex involvement of endogenous opioids in feeding and body weight control. On one hand, that endogenous enkephalins may mediate the incentive motivational properties of food, and support body weight gain, is in line with the notion that enkephalin transmission throughout the body is involved in the homeostasis of energy balance (Levine and Atkinson, 1987). On the other hand, it is difficult to reconcile an expected profeeding role of endogenous β-endorphin with the lack of significant effects observed on weight gain, although others have noted similar paradoxical effects of endogenous β-endorphin (Hayward et al, 2006). Clearly, further study is necessary to resolve this issue; however on an applied level, these observations may shed light on why nonspecific opiate antagonists are generally effective at decreasing feeding acutely (Reid, 1985; Yeomans and Gray, 2002), but have been met with only limited long-term success in the control of body weight (Atkinson et al, 1985; Mitchell et al, 1987; Wright and Rodgers, 2013).
In summary, the results of this study are in line with other studies suggesting that endogenous opioids play a role in regulating consummatory behavior and weight gain during access to palatable diets (Atkinson et al, 1985; Czyzyk et al, 2012; Levine et al, 1995; Ostlund et al, 2013; Reid, 1985). Such results are interesting when viewed in the context of human genetic screens strongly implicating opioid receptors in obesity phenotypes (Haghighi et al, 2013; Wheeler et al, 2013). Additionally, antagonism of opioid receptors can in some circumstances be effective for reversing such phenotypes in preclinical (Giuliano et al, 2012; Shaw et al, 1991; Statnick et al, 2003) and clinical studies (Cambridge et al, 2013; Ziauddeen et al, 2012). Further understanding of the contributions of opioid transmission to hedonic processing and control of body weight may aid in developing appropriate treatment strategies aimed at pathologies characterized by under- or over-eating.
Funding and Disclosure
The authors declare no conflict of interest.
References
Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM (1984). Endogenous opioids: biology and function. Annu Rev Neurosci 7: 223–255.
Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M et al (2003). A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology 144: 1753–1760.
Atkinson RL, Berke LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL (1985). Effects of long-term therapy with naltrexone on body weight in obesity. Clin Pharmacol Ther 38: 419–422.
Bachmanov AA, Tordoff MG, Beauchamp GK (2001). Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses 26: 905–913.
Bakshi VP, Kelley AE (1993). Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology 111: 207–214.
Berridge KC (2009). 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiol Behav 97: 537–550.
Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 1350: 43–64.
Berridge KC, Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369.
Berridge KC, Robinson TE, Aldridge JW (2009). Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol 9: 65–73.
Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML (1995). General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res 700: 205–212.
Cambridge VC, Ziauddeen H, Nathan PJ, Subramaniam N, Dodds C, Chamberlain SR et al (2013). Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol Psychiatry 73: 887–894.
Czyzyk TA, Pico AR, Pintar J, McKinzie JH, Tschop MH, Statnick MA et al (2012). Mice lacking δ-opioid receptors resist the development of diet-induced obesity. FASEB J 26: 3483–3492.
D'Aquila PS (2010). Dopamine on D2-like receptors "reboosts" dopamine D1-like receptor-mediated behavioural activation in rats licking for sucrose. Neuropharmacology 58: 1085–1096.
Davis JD, Smith GP (1988). Analysis of lick rate measures the positive and negative feedback effects of carbohydrates on eating. Appetite 11: 229–238.
DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC (2012). Enkephalin surges in dorsal neostriatum as a signal to eat. Curr Biol 22: 1918–1924.
Doyle TG, Berridge KC, Gosnell BA (1993). Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav 46: 745–749.
Dwyer DM (2012). Licking and liking: the assessment of hedonic responses in rodents. Q J Exp Psychol 65: 371–394.
Farhang B, Pietruszewski L, Lutfy K, Wagner EJ (2010). The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology 59: 190–200.
Frank GK, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT et al (2008). Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 39: 1559–1569.
Frisina PG, Sclafani A (2002). Naltrexone suppresses the late but not early licking response to a palatable sweet solution: opioid hedonic hypothesis reconsidered. Pharmacol Biochem Behav 74: 163–172.
Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W et al (2011). Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci 33: 668–677.
Galistu A, Modde C, Pireddu MC, Franconi F, Serra G, D'Aquila PS (2011). Clozapine increases reward evaluation but not overall ingestive behaviour in rats lickingfor sucrose. Psychopharmacology 216: 411–420.
Genn RF, Higgs S, Cooper SJ (2003). The effects of 7-OH-DPAT, quinpirole and raclopride on licking for sucrose solution in the non-deprived rat. Behav Pharmacol 14: 609–617.
Giraudo SQ, Grace MK, Billington CJ, Levine AS (1999). Differential effects of neuropeptide Y and the mu-agonist DAMGO on 'palatability' vs. 'energy’. Brain Res 834: 160–163.
Giuliano C, Robbins TW, Nathan PJ, Bullmore ET, Everitt BJ (2012). Inhibition of opioid transmission at the μ-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacology 37: 2643–2652.
Gosnell BA, Levine AS (2009). Reward systems and food intake: role of opioids. Int J Obes 33: S54–S58.
Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH (2009). The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9: 88.
Haghighi A, Melka MG, Bernard M, Abrahamowicz M, Leonard GT, Richer L et al (2013). Opioid receptor mu 1 gene, fat intake and obesity in adolescence. Mol Psychiatry 19: 63–68.
Hayward MD, Schaich-Borg A, Pintar JE, Low MJ (2006). Differential involvement of endogenous opioids in sucrose consumption and food reinforcement. Pharmacol Biochem Behav 85: 601–611.
Higgs S, Cooper SJ (1998). Evidence for early opioid modulation of licking responses to sucrose and intralipid: a microstructural analysis in the rat. Psychopharmacology 139: 342–355.
Jiang ZG, North RA (1992). Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci 12: 356–361.
Kelley AE, Baldo BA, Pratt WE, Will MJ (2005). Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol Behav 86: 773–795.
König M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ et al (1996). Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 383: 535–538.
Laurent V, Bertran-Gonzalez J, Chieng BC, Balleine BW (2014). δ-opioid and dopaminergic processes in accumbens shell modulate the cholinergic control of predictive learning and choice. J Neurosci 34: 1358–1369.
Laurent V, Leung B, Maidment N, Balleine BW (2012). mu- and delta-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice. J Neurosci 32: 1875–1883.
Le Merrer J, Becker JAJ, Befort K, Kieffer BL (2009). Reward processing by the opioid system in the brain. Physiol Rev 89: 1379–1412.
Levine AS, Atkinson RL (1987). Opioids in the regulation of food intake and energy expenditure. Fed Proc 46: 159–162.
Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ (1995). Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol 268: R248–R252.
Liao RM, Ko MC (1995). Chronic effect of haloperidol and SCH23390 on operant and licking behaviors in the rat. Chin J Physiol 38: 65–73.
Mahler SV, Berridge KC (2009). Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29: 6500–6513.
Mena JD, Sadeghian K, Baldo BA (2011). Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci 31: 3249–3260.
Millington GWM (2007). The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab 4: 18.
Mitchell JE, Morley JE, Levine AS, Hatsukami D, Gannon M, Pfohl D (1987). High-dose naltrexone therapy and dietary counseling for obesity. Biol Psychiatry 22: 35–42.
Murphy NP (2015). Dynamic measurement of extracellular opioid activity: status quo,challenges, and significance in rewarded behaviors. ACS Chem Neurosci 6: 94–107.
Nogueiras R, Romero-Pico A, Vazquez MJ, Novelle MG, Lopez M, Dieguez C (2012). The opioid system and food intake: homeostatic and hedonic mechanisms. Obes Facts 5: 196–207.
Ostlund SB, Kosheleff A, Maidment NT, Murphy NP (2013). Decreased consumption of sweet fluids in mu opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology 229: 105–113.
Pecina S, Smith KS (2010). Hedonic and motivational roles of opioids in food reward:implications for overeating disorders. Pharmacol Biochem Behav 97: 34–46.
Reid LD (1985). Endogenous opioid peptides and regulation of drinking and feeding. Am J Clin Nutr 42: 1099–1132.
Rubinstein M, Mogil JS, Japón M, Chan EC, Allen RG, Low MJ (1996). Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci USA 93: 3995–4000.
Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT et al (2011). Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity 19: 1109–1117.
Schneider LH, Davis JD, Watson CA, Smith GP (1990). Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol 186: 61–70.
Shaw WN, Mitch CH, Leander JD, Mendelsohn IG, Zimmerman DM (1991). The effect of the opioid antagonist LY255582 on body weight of the obese Zucker rat. Int J Obes 15: 387–395.
Smith GP (2004). Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite 43: 11–13.
Statnick MA, Tinsley FC, Eastwood BJ, Suter TM, Mitch CH, Heiman ML (2003). Peptides that regulate food intake: antagonism of opioid receptors reduces body fat in obese rats by decreasing food intake and stimulating lipid utilization. Am J Physiol Regul Integr Comp Physiol 284: R1399–R1408.
Tabarin A, Chaves YD, Carmona MDC, Catargi B, Zorrila EP, Roberts AJ et al (2005a). Resistance to diet-induced obesity in μ-opioid receptor-deficient mice. Diabetes 54: 3510–3516.
Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ et al (2005b). Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a "thrifty gene". Diabetes 54: 3510–3516.
Wassum KM, Cely IC, Balleine BW, Maidment NT (2011). Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but notdecreases in the incentive value of a food reward. J Neurosci 31: 1591–1599.
Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S et al (2013). Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet 45: 513–517.
Wright FL, Rodgers RJ (2013). Acute behavioural effects of bupropion and naltrexone, alone and in combination, in non-deprived male rats presented with palatable mash. Psychopharmacology 228: 291–307.
Yeomans MR, Blundell JE, Leshem M (2004). Palatability: response to nutritional need orneed-free stimulation of appetite? Br J Nutr 92 (Suppl 1): S3–14.
Yeomans MR, Gray RW (2002). Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev 26: 713–728.
Zhang M, Gosnell BA, Kelley AE (1998). Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285: 908–914.
Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M et al (2012). Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry 18: 1287–1293.
Zuberi AR, Townsend L, Patterson L, Zheng H, Berthoud HR (2008). Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in μ-opioid receptor-deficient mice. Eur J Pharmacol 585: 14–23.
Acknowledgements
This work was supported by NIDA grants DA024635 to IAM, DA005010 to NTM, and DA029035 to SBO, and by the Shirley and Stefan Hatos Neuroscience Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Mendez, I., Ostlund, S., Maidment, N. et al. Involvement of Endogenous Enkephalins and β-Endorphin in Feeding and Diet-Induced Obesity. Neuropsychopharmacol 40, 2103–2112 (2015). https://doi.org/10.1038/npp.2015.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.67
This article is cited by
-
Lateral hypothalamic proenkephalin neurons drive threat-induced overeating associated with a negative emotional state
Nature Communications (2023)
-
Early adversity promotes binge-like eating habits by remodeling a leptin-responsive lateral hypothalamus–brainstem pathway
Nature Neuroscience (2023)
-
The immune-opioid axis in prediabetes: predicting prediabetes with insulin resistance by plasma interleukin-10 and endomorphin-2 to kappa-opioid receptors ratio
Diabetology & Metabolic Syndrome (2021)
-
Connections of the mouse subfornical region of the lateral hypothalamus (LHsf)
Brain Structure and Function (2021)
-
Contributions of Pavlovian incentive motivation to cue-potentiated feeding
Scientific Reports (2018)