Abstract
Varenicline, a nicotinic partial agonist, is the most effective treatment for tobacco use disorder. However, its mechanism of action is still unclear and may involve stimulating dopaminergic transmission. Here we used PET imaging with [11C]-(+)-PHNO to explore for the first time the impact of varenicline on dopamine transmission in the D2-rich striatum and D3-rich extra-striatal regions and its relationship with craving, withdrawal and smoking. Eleven treatment-seeking smokers underwent two PET scans with [11C]-(+)-PHNO, each following 12-h overnight smoking abstinence both prior to receiving varenicline and following 10–11 days of varenicline treatment (ie, at steady-state drug levels). Subjective measures of craving and urges to smoke were also assessed on the days of the PET scans. Varenicline treatment significantly reduced [11C]-(+)-PHNO binding in the dorsal caudate (p=0.008) and reduced some craving measures. These findings provide the first evidence that varenicline is able to increase DA levels in the human brain, a factor that may contribute to its therapeutic efficacy.
Similar content being viewed by others
INTRODUCTION
Smoking is a public health concern, yet pharmacological treatment strategies remain only partly effective. Current first-line medications include nicotine replacement therapy (NRT) and prescription medications such as bupropion and varenicline, with moderate effect sizes (Cahill et al, 2012). NRT reduces acute nicotine withdrawal while bupropion and varenicline appear to decrease the urge to smoke by reducing withdrawal symptoms and blunting the rewarding effects of smoking (Brandon et al, 2011; Gonzales et al, 2006; Jorenby et al, 2006; Patterson et al, 2009). Exploring the neurobiological underpinning of these treatments is warranted as they are poorly understood in humans.
Dopamine (DA) is a neurotransmitter believed to be important in the final common path in drug dependence, including nicotine (Pich et al, 1997). DA is believed to mediate both the rewarding (Brody et al, 2004; Corrigall et al, 1992; Le Foll et al, 2014) and withdrawal-associated (Rada et al, 2001; Rahman et al, 2004) effects of nicotine. That is, increased DA is associated with ratings of positive subjective measures (Montgomery et al, 2007), and decreased DA is believed to mediate the negative state during withdrawal (Hildebrand et al, 1998; Le Foll et al, 2014). As a partial agonist of the α4β2* acetylcholine nicotinic receptor (Coe et al, 2005), it is possible that varenicline-induced elevations in DA levels may contribute to its therapeutic effects (Coe et al, 2005). However, the first study to evaluate the impact of subchronic administration of varenicline in animals that were not dependent on nicotine found no effect of varenicline on basal DA levels but an ability of varenicline to decrease nicotine-induced DA release in animals (Ericson et al, 2009). More recently, it has been reported that varenicline increased DA firing rates in nicotine-dependent animals that were in acute withdrawal (Perez et al, 2015), an effect consistent with its partial agonist profile. To date, the impact of varenicline on DA transmission in the human brain has not been reported.
Neuroreceptor imaging techniques with PET provide a method to investigate changes in DA in the human brain in vivo (Laruelle, 2000; Laruelle et al, 2002; Martinez and Narendran, 2010). The traditional radioligand for dopamine D2/3 receptors (DRD2/3) is [11C]-raclopride that has relatively low sensitivity to detect changes in DA (Martinez and Narendran, 2010). However, [11C]-(+)-PHNO (Wilson et al, 2005) is a DRD2/3 agonist that is also the most sensitive PET tracer to detect relatively small fluctuations in DA (Gallezot et al, 2012; Ginovart et al, 2006, 2007; Narendran et al, 2006; Willeit et al, 2006). We have recently shown that [11C]-(+)-PHNO has an enhanced ability to detect elevations in DA induced by smoking (Le Foll et al, 2014), with a preferential effect in the limbic striatum. In addition, [11C]-(+)-PHNO allows for the measurement of occupancy of DA receptors in D2- and D3-rich areas. [11C]-(+)-PHNO binding in the substantia nigra (SN) and ventral pallidum (VP) is believed to represent 100 and 75% binding to DRD3, respectively, while [11C]-(+)-PHNO binding in the dorsal caudate and dorsal putamen is accounted for by DRD2, and the VST is about 50% of each (Ginovart et al, 2007; Rabiner et al, 2009; Tziortzi et al, 2011).
The purpose of the present study was to evaluate whether treatment with varenicline affects DA transmission in the D2-rich striatum and D3-rich extra-striatal regions and whether this change was associated with changes in subjective measures. Participants underwent two PET scans with [11C]-(+)-PHNO after overnight abstinence from tobacco. The first PET scan was done before varenicline treatment and the second PET scan was done after 10–11 days of varenicline (at steady state). Subjective questionnaires that measured craving and reward were administered, and objective measures (ie, varenicline and plasma cotinine) were collected.
MATERIALS AND METHODS
Participants
All procedures were approved by the Centre for Addiction and Mental Health (CAMH) Research Ethics Board and complied with the Helsinki Declaration of 1975 (as revised in 1983). The study was approved by the Institutional Research Ethics Board. Thirteen participants (adult males or females 21–45 years) were recruited from the community and provided written informed consent prior to participating in any study procedures. All met the following inclusion criteria: (1) Nicotine dependent as assessed by smoking at least 10 cigarettes a day, a baseline score of ⩾4 on the Fagerstrom Test of Nicotine Dependence and expired carbon monoxide (CO) levels of at least 10 p.p.m.; (2) motivated to quit within the next 30 days; and (3) treatment-seekers who were willing to use varenicline as a quit aid. Exclusion criteria were: (1) Previous use of medication for smoking cessation within the past month; (2) Abnormal physical examination, 12-lead or routine routine blood tests or a condition that may impede memory and attention; (3) Past/present axis I psychiatric diagnoses as per MINI-International Neuropsychiatric Interview version 5.0 and the Hamilton Depression Rating Scale; (4) Magnetic resonance (MR) scanning contraindication; (5) Claustrophobia; (6) Current pregnancy/breastfeeding; (7) Current use or use during the previous month of medication that may affect the central nervous system or positive during drug screening for drugs of abuse or abuse of alcohol or drugs of abuse within the past 3 months; (8) Exposure to radiation in the past 12 months exceeding limits for participants in research with PET; and (9) Allergy to varenicline.
Procedure
PET/MRI scans
After enrolment in the study, participants had two PET scans and a 30-min MRI on a 3 Tesla GE MRI scanner (Discovery MR750, GE, Milwaukee, USA) for region of interest (ROI) delineation. On the day of the first PET scan, participants were scanned after overnight (12 h) abstinence from tobacco and given varenicline to take home, with the instructions to start taking the medication the next day (see dosing paradigm below). Seven days later, participants returned to collect refills of blister packs of varenicline and the second PET day was scheduled 10–11 days after the first PET day, after reaching maintenance doses of varenicline. Participants were asked to refrain from smoking for 12 h prior to each PET visit, and smoking abstinence was confirmed by breath CO levels below 10 p.p.m. Alcohol abstinence was also confirmed by a breath alcohol measure. At the start of each PET scan, a sample of blood was drawn to measure plasma levels of varenicline, cotinine, and nicotine. Participants completing the second PET scan were followed every 2 weeks for a total duration of 12 weeks for treatment of tobacco dependence. During follow-up visits, subjects received behavioral support adapted from the manual from the Mayo Clinic Guide (Smoke Free and Livin'it), were given blister packs of medication, and provided breath CO and completed questionnaires (see below). A final visit was scheduled 3 months after varenicline was discontinued.
Subjective measures
During each PET scan visit, participants completed the Minnesota Nicotine Withdrawal Scale (MNWS; assesses urge to smoke, depressed mood, irritability, anxiety, difficulty concentrating, restlessness, increased appetite, and sleep) and the Tobacco Craving Questionnaire (TCQ; relief from withdrawal (factor 1), anticipation of positive outcomes (factor 2), control over tobacco use (factor 3) and intention to smoke for positive outcomes (factor 4) both before and after each PET scan). The patient health questionnaire (PHQ-9; to assess depressive symptoms) was also administered on each PET scan day. All these questionnaires were also given on day 7 and on the follow-up visits with the exception of the TCQ. Participants kept a daily log of the number of cigarettes smoked. At each visit, participants were asked to indicate how many cigarettes they had smoked on each day for the 7 days prior to the visit, and an expired breath CO reading was taken. Participants were also asked how much alcohol and caffeine they had during the past 7 days.
Drug administration
Varenicline was administered as prescribed in clinical practice. For the first 3 days, participants took 0.5 mg orally once a day in the morning and then twice a day on days 4–7. After that, 1 mg varenicline was taken orally twice a day. The target quit date was set at the second PET scan visit (days 10–11 of taking varenicline). Concomitant medications and adverse events were assessed at each visit.
PET Image Acquisition
The radiosynthesis of [11C]-(+)-PHNO has been described in detail elsewhere (Wilson et al, 2005). PET scans were performed using a Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN, USA) PET/CT camera system, which measures radioactivity in 81 brain sections with a reconstructed pixel size of 1.07 × 1.07 × 2.00 mm3 each with an in-plane resolution of 5 mm full-width at half maximum. A transmission scan was acquired and the emission scan, acquired in 32-bit list mode, began after bolus injection of [11C]-(þ)-PHNO (duration of the bolus injection approximately 2 min). Emission data were reconstructed by 2D filtered back projection to yield dynamic images with 15 1-min frames and 15 5-min frames. The emission scan lasted for 90 min. The raw data were reconstructed by filtered-back projection. A custom-fitted thermoplastic mask (Tru-Scan Imaging, USA) was made for each subject to reduce movement during the acquisition. A total of ~370±40 MBq (approximately 10±1 mCi) of [11C]-(+)-PHNO was injected as a bolus into an antecubital vein.
Plasma Levels of Varenicline, Nicotine and Cotinine
Consistent with its elimination half-life of approximately 24 h, steady-state conditions for varenicline are reached within 4 days of repeat dosing (Faessel et al, 2010). Plasma levels of nicotine, cotinine and varenicline, were measured by LC/MS/MS using previously established methods (St Helen et al, 2012; Tanner et al, 2015) for nicotine, cotinine and 3-hydroxycotinine modified to additionally detect varenicline. Varenicline measured by this method provided identical levels as observed using other methods.
PET Image Analysis
ROI delineation and time activity curve analyses were performed using ROMI (details in (Rusjan et al, 2006). ROI included the dorsal caudate (DC), dorsal putamen (DP), ventral striatum (VST) as well as globus pallidus (GP; whole), VP, and SN. Delineation is described elsewhere (Boileau et al, 2012). [11C]-(+)-PHNO specific binding (BPND) was estimated in each ROI using the simplified reference tissue method (SRTM; Lammertsma and Hume, 1996), with cerebellar cortex (excluding vermis) as reference region. Parameter estimation was performed using PMOD (Version 2.8.5; PMOD Technologies, Zurich, Switzerland). Percentage of occupancy was calculated as [11C]-(+)-PHNO binding after varenicline/([11C]-(+)-PHNO binding at baseline −1).
Voxel-wise parameter estimation of [11C]-(+)-PHNO binding was generated using the basis function implementation of SRTM (Lammertsma and Hume, 1996), with the tissue time activity curve of cerebellar cortex as the reference region. Normalized BPND maps (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK) were statistically investigated to assess significant contrasts between conditions at every voxel using paired sample t-test analysis. The threshold for significant clusters was set to a family-wise error corrected p=0.05.
Comparisons between [11C]-(+)-PHNO BPND in ROIs were conducted by using repeated-measures Day (two levels; baseline, post-varenicline) × Region (six levels; ROIs: SN, GP, VP, VST, DC, DP) ANOVAs (SPSS 20.0, SPSS, USA). Sphericity was assessed with Mauchly test, and corrections were made when indicated. Bonferonni-corrected paired t-tests were conducted between the baseline scan and scan under varenicline for each ROI.
Subjective questionnaire data obtained prior to and following each PET scan were averaged for each PET scan day. Questionnaires were analyzed with Day (two levels; baseline PET scan days vs PET scan after 11 days of treatment with varenicline) × Measure (six levels; TCQ1, TCQ2, TCQ3, TCQ4, MNWS, and PHQ) ANOVAs. All objective measures (number of cigarettes smoked and plasma cotinine) were analyzed with ANOVAs on the effect of Day (two levels). Correlations between questionnaire measures and BPND were conducted for any ROIs that had any significant changes in BPND.
RESULTS
Thirteen participants were recruited for this study. One participant withdrew after the first scan. For another participant, there was a movement artifact in the PET scan that prevented proper determination of [11C]-(+)-PHNO BPND, and data were not included. A total of 11 subjects were included in the final analysis.
All participants tested negative for drugs of abuse at the time of scanning. Varenicline was detected in all participants on the day of second PET scan. Demographic variables are provided in Table 1. A mixed ANOVA of Measure (mass injected, amount injected, specific activity) × Day (PET baseline, PET after varenicline) revealed no effects, suggesting that there were no differences in scan parameters across scans or between groups (mass injected (μg) 2.09±0.09; amount injected (mCi) 9.45±0.25; specific activity (mCi/μmol) 1695.59±99.81).
Changes in Binding Potential
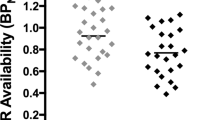
A two-way repeated-measures Region (six levels) × Day (two levels) revealed no interaction and no effect of Day. t-Tests corrected for multiple comparisons (Bonferroni) indicated that BPND differed between baseline and while taking varenicline only in the DC (Figure 1; p=0.008; Bonferroni uncorrected to 0.0083; Cohen’s D: VST: 0.168; VP: 0.41; SN: 0; GP: 0.53; DC: 0.49; DP: 0.20).
Mean±SEM [11C]-(+)-PHNO BPND before varenicline (open bars) and after 10–11 days of varenicline (filled bars) presented for individual participants for ROIs (DC, dorsal caudate; DP, dorsal putamen: GP, globus pallidus; SN, substantia nigra; VP, ventral pallidum; VST, ventral striatum). [11C]-(+)-PHNO BPND was significantly decreased in the DC after treatment with varenicline (*p<0.05, paired t-tests).
Voxel-Wise Analyses
In the voxel-wise statistical analyses (SPM8), we identified small clusters of lower [11C]-(+)-PHNO BPND in the varenicline condition as compared with baseline in areas corresponding to the ventral lateral nucleus of the thalamus and the head/body of the caudate nucleus (on left voxel 48 with peak threshold of 4.15 and on the right a cluster of 57 voxels with a peak threshold of 4.01; Figure 2). No other clusters of significantly lower [11C]-(+)-PHNO BPND was significant after correction for multiple comparisons.
Voxel-wise comparison between baseline and varenicline illustrating lower [11C]-(+)-PHNO BPND in the DC (tmax=5.92; p=0.05). The image is not corrected for multiple comparisons.
Objective and Subjective Measures
On the first PET session, prior to taking varencline, the number of cigarettes smoked in the 7 days prior was 98±12. On the day of the second PET scan, after taking varenicline for 11 days, the number of cigarettes smoked in the 7 days prior was 93±8. A one-way ANOVA revealed no significant effect of Day for the objective measures of number of cigarettes smoked or cotinine levels (day 1: 210=42; day 11: 149±29). A two-way repeated-measures Day (two levels) × Measure (six levels; TCQ1, TCQ2, TCQ3, TCQ4, MNWS, PHQ) ANOVA revealed a significant interaction (F(5,45)=5.040, p=0.001). Main effects of Day were found for TCQ2 (F(1,9)=10.229, p<0.011) and TCQ4 (F(1,10)=3.494, p=0.091). For the MNWS and PHQ, main effects of Day approached significance (MNWS: F(1,10)=3.564, p=0.088; PHQ: F(1,10)=3.494, p=0.091). Bonferroni-corrected t-tests (corrected p=0.0083) revealed differences between the 2 days only for TCQ factor 4 (p=0.006), suggesting that the intention and planning to smoke for positive outcomes was reduced after varenicline. The effect of Day for TCQ factor 2 was not statistically significant after correction for multiple comparisons (p=0.011, see Figure 3). One participant was missing subjective data from day 1 and was not included in this analysis.
Mean±SEM scores on the Tobacco Craving Questionnaire (TCQ) factors 1–4 and the Minnesota Nicotine Withdrawal Scale (MNWS) presented before (open squares) and after (closed squares) treatment with varenicline. *Significant effects of treatment with varenicline were found in the scores on the TCQ factor 4 (intention and planning to smoke for positive outcomes; t-test, p=0.006). TCQ factor 2 (anticipation of positive outcomes) was significant but did not survive corrections for multiple comparisons (paired t-test: p=0.011; Bonferroni p=0.0083).
Correlations
Correlations (Figure 4) between changes in BPND in the DC and subjective measures (PHQ, TCQ1, TCQ2, TCQ3, TCQ4, MNWS) revealed that the correlation between changes in BPND in the DC and changes in TCQ factor 2 (anticipation of positive outcomes) approached, but did not reach, significance (r2=−0.505, p=0.136). One participant was missing subjective data from day 1 and was not included in the analyses. A significant correlation between change in plasma cotinine and change in BPND in the DC was found (r2=−0.742, p=0.009)
Correlations changes in TCQ factor 2 and changes in BPND in the DC approached significance. Improvements in the TCQ score were correlated with decreased BPND after varenicline (ie, increased DA).
DISCUSSION
The purpose of the present study was to investigate the effects of varenicline treatment on [11C]-(+)-PHNO BPND in ROIs in treatment-seeking smokers. PET scans were performed before and after 10–11 days of varenicline treatment (at steady state). Subjective mood and craving questionnaires as well objective measures of plasma levels of varenicline and cotinine were also taken. After treatment with varenicline, [11C]-(+)-PHNO BPND was significantly decreased in the DC, as assessed with voxel-wise and ROI analyses. Subjective measures of TCQ factor 4 (intention to smoke for positive outcomes) were also decreased, while TCQ factor 2 (anticipation of positive outcomes from smoking) failed to reach significance after correction for multiple comparisons.
This study revealed that [11C]-(+)-PHNO BPND was lower in DC after treatment with varenicline, as compared with baseline in treatment-seeking smokers. Changes in BPND could be obtained not only by changes in DA but possibly also by changes in DA receptor expression. However, the latter is unlikely here as preclinical studies suggest that if varenicline has any effects on DA receptor expression it would be an increase in receptor number (Crunelle et al, 2012), which would then be producing an increase in [11C]-(+)-PHNO binding. Therefore, it is more likely that the lowest [11C]-(+)-PHNO BPND reflects an elevation of DA in DC. This effect was consistently found with both our ROI analysis and with the voxel-wise analysis approach. The voxel-wise approach is aimed at detecting differences in neuroreceptor ligand binding at the voxel level, with no a priori anatomical hypothesis, and enables circumvention of some limitations of ROI placement, as well as investigation of regions not included in our ROI template. It should be noted that, in this study, participants were abstinent from tobacco use, in order to avoid the confound of tobacco-induced elevations in DA (Le Foll et al, 2014).
Reported intention to smoke for the positive effects of tobacco (TCQ factor 4) were decreased. This decrease in the positive effects of smoking is consistent with both preclinical (George et al, 2011; Le Foll et al, 2012) and clinical (Gonzales et al, 2006; Jorenby et al, 2006) studies that have demonstrated that varenicline affects the rewarding properties of smoking. Given that positive reactions to smoking have been shown to predict relapse (Strong et al, 2011), decreases in measures of positive reinforcement may provide an explanation as to the efficacy of varenicline (Gonzales et al, 2006; Jorenby et al, 2006). In addition, varenicline also decreased the negative reinforcement of withdrawal (Gonzales et al, 2006; Jorenby et al, 2006). However, changes in brain DA and subjective appraisals of smoking occurred in the absence of any changes in plasma cotinine or the number of cigarettes smoked per day from baseline. Thus changes in subjective values or BPND do not reflect alterations in smoking habits per se. This is compelling as it suggests that varenicline may act to alter the brain and subjective response prior to quitting, thus enabling the mechanism by which smokers may subsequently quit. It may also explain the delayed quitting observed up to 4 weeks after taking varenicline (Agboola et al, 2010, 2015; Kasza et al, 2013). It has been shown that treatment with the nicotine patch prior to the quit date can improve smoking cessation (Rose et al, 2009); in smokers who did not decrease smoking prior to their quit date while undergoing nicotine replacement therapy, they could be ‘rescued’ by bupropion augmentation of the patch or with varenicline treatment alone (Rose and Behm, 2013). Future studies can address how brain response and subjective measures can predict the success of smoking cessation interventions.
Varenicline and tobacco smoke affect DA differently. Varenicline appears to produce elevations in DA in a D2-rich areas (DC) but not in D3-rich areas (SN, VP). In contrast, tobacco produced elevations in both limbic striatum and in D3-rich area (VP), but not in the DC, as assessed with [11C]-(+)-PHNO (Le Foll et al, 2014). These differential effects may be due to the different pharmacological agonist properties (full agonist for nicotine vs partial agonist for varenicline) or the involvement of different subtypes of nicotinic acetylcholine receptors (Coe et al, 2005). Another possibility is that varenicline predominantly stimulated nicotinic acetylcholine receptors located in DA neurons that preferentially project to DC. Further studies should explore for such effects. Regardless of the receptor target (D2 or D3), [11C]-(+)-PHNO has been shown to have a greater sensitivity in detecting smaller changes in synaptic DA levels as compared with [11C]-raclopride (Ginovart et al, 2007; Narendran et al, 2006; Willeit et al, 2006). This is supported by the direct comparison of the dose–effect of amphetamine (0.1, 0.5, and 2 mg/kg; i.v.) on binding of [11C]-(+)-PHNO and [11C]-raclopride in cats (Ginovart et al, 2006) and humans (Shotbolt et al, 2012).
To our knowledge, no study has investigated the effects of bupropion or NRT using [11C]-(+)-PHNO. Previous studies measuring the impact of bupropion on DA transmission using [11C]-raclopride found no effect on ventral caudate/nucleus accumbens binding induced by bupropion (Brody et al, 2010). Limited work has been carried out assessing the impact of NRT on dopamine transmission with PET. Nicotine gum (Takahashi et al, 2008), but not nicotine spray (Montgomery et al, 2007), was effective in decreasing [11C]-raclopride in striatal area. As compared with the traditional radiotracer [11C]-raclopride, [11C]-(+)-PHNO allows for greater sensitivity in the measurement of change in DA levels (Gallezot et al, 2012; Ginovart et al, 2006, 2007; Narendran et al, 2006; Willeit et al, 2006). It is possible that our ability to detectsignificant elevations in DA induced by varenicline was due to the use of [11C]-(+)-PHNO vs [11C]-raclopride. As response to treatment for varenicline and for NRT has been related to nicotine metabolic rate (Lerman et al, 2015), further studies exploring the impact of nicotine metabolic rate on [11C]-(+)-PHNO would be informative.
Partial agonists are believed to be able to decrease drug-induced elevations in DA and also to elevate DA levels during withdrawal (Childress and O'Brien, 2000). Therefore, increases in DA levels in the DC in the present study may reflect a reversal of attenuated DA levels normally seen during withdrawal. It should be noted that the present study did not measure whether varenicline could decrease elevations in DA levels induced by smoking in humans. Further studies could explore this as a further test of the partial agonist properties of varenicline.
This study has several limitations. First, the study has a limited sample size. However, despite this limited sample size, we were able to detect significant changes in [11C]-(+)-PHNO BPND in the DC that were still significant after corrections for multiple testing. But it is possible that with a larger sample size we would have detected an effect of varenicline in more brain areas and also had more power to detect changes in subjective measures. Indeed, previous studies have found alterations in measures of withdrawal following varenicline (Gonzales et al, 2006; Jorenby et al, 2006). Further, we were not able to analyze the data in terms of those who respond to treatment compared with those who do not respond to treatment (only three responded to treatment). Future investigations may reveal the predictive relationship between early changes in BPND and response to treatment in smokers, as already identified in subjects with cocaine use disorders undergoing behavioral treatment (Martinez et al, 2011).
Related to the relatively small sample size is the inability in the present study to look at individual differences. One of these is gender effects (Cosgrove et al, 2014). It has been shown, using [11C]raclopride, that DA levels in the ventral striatum are increased in males as compared with females during cigarette smoking. In the present study, the sample consisted of seven males and four females, and thus the data may disproportionately represent one gender, with not enough power to compare the two. It is noteworthy that effects found in the present study in the VST were minimal (Cohen’s D of 0.168), and thus it is possible that gender effects are more pronounced during smoking than withdrawal per se, as studied in the present study. Future studies will need to determine whether gender differences exist in the neurochemical response to treatment approaches for smoking cessation.
Further, there are some limitations owing to the PET scanning parameters. The injected mass of the radiotracer was slightly above the limit suggested by others (Gallezot et al, 2012), potentially leading to underestimation of DA occupancy in both conditions (Shotbolt et al, 2012). The fact that the mass injected was similar in the two conditions suggests that it did not interfere with the results. Although our analysis based on the brain area suggests that varenicline has an impact predominantly at the level of the DRD2 receptor in the DC, we do not have a clear explanation as to why there would be no effect at the level of the DRD3. In our previous studies, we have found that DRD3 levels are increased in the brains of people with psychostimulant use disorder (Boileau et al, 2012; Payer et al, 2013).
It should be considered, given the lack of a control group, that the present results may be due, perhaps in part, to a placebo effect. The placebo effect is common in the treatment of pain, depression, and Parkinson’s disease and is set up by an expectation on the part of the participant that a treatment will be successful (for reviews, see Lidstone and Stoessl, 2007; Murray and Stoessl, 2013). Particularly powerful in establishing a placebo effect are environmental considerations such as a hospital and controlled clinical trial setting, both of which were true here. With the use of [11C]raclopride, it has been shown that the placebo effect can be associated with increases in DA in the dorsal and ventral striatum, the same brain regions that are involved in reward expectation (de la Fuente-Fernandez et al, 2001, 2002, 2006; de la Fuente-Fernandez and Stoessl, 2002; Strafella et al, 2001, 2003, 2006). Given the increase in DA observed in the DC in the present study, the possibility that this may be partly explained by a placebo effect cannot be ruled out. However, this suggestion is somewhat tempered by the fact the placebo effect also elevates DA in the VST, something that was not found in the present study.
CONCLUSIONS
The purpose of the present study was to determine whether steady-state levels of varenicline can increase DA levels in the brain of abstinent smokers. It was found that DA was increased in the DC after treatment with varenicline and that this increase was (nearly) correlated with decreases in ratings of the positive effects of smoking. These findings indicate for the first time that varenicline increases DA transmission in human smokers scanned under abstinence. This effect may contribute to its therapeutic efficacy, and future studies would be needed to determine this relationship, especially as varenicline negatively affects positive ratings of smoking (Agboola et al, 2010, 2015; Cahill et al, 2012).
FUNDING AND DISCLOSURE
In the past 3 years, RFT has consulted for Apotex on issues unrelated to smoking. In the previous 3 years, PS has received funding from the Centre for Addiction and Mental Health, Canadian Cancer Society, Canadian Institutes of Health Research, Health Canada, the Association of Faculties of Medicine of Canada, Ontario Ministry of Health and Long-Term Care, Ontario Brain Institute, Ontario Lung Association, Cancer Care Ontario, Ontario Institute for Cancer Research, National Institutes of Health, Workplace Safety and Insurance Board, Pfizer, McLaughlin Centre for Molecular Medicine, and Shoppers Drug Mart. BLF has received support unrelated to the current project from Pfizer, Bioprojet, and Mettrum and various public funding agenices. This work was funded in part by an Ontario Lung Association-Pfizer grant awarded to BLF and a CIHR grant TMH-109787 to RFT. We acknowledge the support of the Endowed Chair in Addictions for the Department of Psychiatry (to RFT), the Campbell Family Mental Health Research Institute of CAMH, the CAMH Foundation, the Canada Foundation for Innovation (nos. 20289 and 16014), and the Ontario Ministry of Research and Innovation. We also acknowledge the support of the Clinician Scientist Salary support Program from the Department of Family and Community Medicine (to PS). The other authors declare no conflict of interest.
References
Agboola S, McNeill A, Coleman T, Leonardi Bee J (2010). A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction 105: 1362–1380.
Agboola SA, Coleman T, McNeill A, Leonardi-Bee J (2015). Abstinence and relapse among smokers who use varenicline in a quit attempt-a pooled analysis of randomized controlled trials. Addiction 110: 1182–1193.
Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J et al (2012). Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci 32: 1353–1359.
Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC et al (2011). Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 218: 391–403.
Brody AL, London ED, Olmstead RE, Allen-Martinez Z, Shulenberger S, Costello MR et al (2010). Smoking-induced change in intrasynaptic dopamine concentration: effect of treatment for Tobacco Dependence. Psychiatry Res 183: 218–224.
Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P et al (2004). Smoking-induced ventral striatum dopamine release. Am J Psychiatry 161: 1211–1218.
Cahill K, Stead LF, Lancaster T (2012). Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 4: CD006103.
Childress AR, O'Brien CP (2000). Dopamine receptor partial agonists could address the duality of cocaine craving. Trends Pharmacol Sci 21: 6–9.
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J et al (2005). Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48: 3474–3477.
Corrigall WA, Franklin KB, Coen KM, Clarke PB (1992). The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107: 285–289.
Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H et al (2014). Sex differences in the brain's dopamine signature of cigarette smoking. J Neurosci 34: 16851–16855.
Crunelle CL, de Wit TC, de Bruin K, Ramakers RM, van der Have F, Beekman FJ et al (2012). Varenicline increases in vivo striatal dopamine D(2/3) receptor binding: an ultra-high-resolution pinhole [(123)I]IBZM SPECT study in rats. Nucl Med Biol 39: 640–644.
de la Fuente-Fernandez R, Lidstone S, Stoessl AJ (2006). Placebo effect and dopamine release. J Neural Transm Suppl 415–418.
de la Fuente-Fernandez R, Phillips AG, Zamburlini M, Sossi V, Calne DB, Ruth TJ et al (2002). Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res 136: 359–363.
de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ (2001). Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 293: 1164–1166.
de la Fuente-Fernandez R, Stoessl AJ (2002). The placebo effect in Parkinson's disease. Trends Neurosci 25: 302–306.
Ericson M, Lof E, Stomberg R, Soderpalm B (2009). The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther 329: 225–230.
Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH (2010). A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet 49: 799–816.
Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T et al (2012). Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse 66: 489–500.
George O, Lloyd A, Carroll FI, Damaj MI, Koob GF (2011). Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl) 213: 715–722.
Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P et al (2006). Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 97: 1089–1103.
Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S et al (2007). Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 27: 857–871.
Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB et al (2006). Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296: 47–55.
Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH (1998). Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res 779: 214–225.
Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE et al (2006). Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama 296: 56–63.
Kasza KA, Hyland AJ, Borland R, McNeill AD, Bansal-Travers M, Fix BV et al (2013). Effectiveness of stop-smoking medications: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction 108: 193–202.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4: 153–158.
Laruelle M (2000). Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20: 423–451.
Laruelle M, Slifstein M, Huang Y (2002). Positron emission tomography: imaging and quantification of neurotransporter availability. Methods 27: 287–299.
Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I et al (2012). Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol 15: 1–10.
Le Foll B, Guranda M, Wilson AA, Houle S, Rusjan PM, Wing VC et al (2014). Elevation of dopamine induced by cigarette smoking: novel insights from a [11C]-+-PHNO PET study in humans. Neuropsychopharmacology 39: 415–424.
Lerman C, Schnoll RA, Hawk LW Jr, Cinciripini P, George TP, Wileyto EP et al (2015). Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med 3: 131–138.
Lidstone SC, Stoessl AJ (2007). Understanding the placebo effect: contributions from neuroimaging. Mol Imaging Biol 9: 176–185.
Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC et al (2011). Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 168: 634–641.
Martinez D, Narendran R (2010). Imaging neurotransmitter release by drugs of abuse. Curr Top Behav Neurosci 3: 219–245.
Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM (2007). The effect of nicotine on striatal dopamine release in man: a [11C]raclopride PET study. Synapse 61: 637–645.
Murray D, Stoessl AJ (2013). Mechanisms and therapeutic implications of the placebo effect in neurological and psychiatric conditions. Pharmacol Ther 140: 306–318.
Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E et al (2006). Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse 60: 485–495.
Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC et al (2009). Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry 65: 144–149.
Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM et al (2013). Heightened D dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [C]-(+)-PHNO. Neuropsychopharmacology 39: 311–318.
Perez XA, Khroyan TV, McIntosh JM, Quik M (2015). Varenicline enhances dopamine release facilitation more than nicotine after long-term nicotine treatment and withdrawal. Pharmacol Res Perspect 3: e00105.
Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C (1997). Common neural substrates for the addictive properties of nicotine and cocaine. Science 275: 83–86.
Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R et al (2009). In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse 63: 782–793.
Rada P, Jensen K, Hoebel BG (2001). Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 157: 105–110.
Rahman S, Zhang J, Engleman EA, Corrigall WA (2004). Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience 129: 415–424.
Rose JE, Behm FM (2013). Adapting smoking cessation treatment according to initial response to precessation nicotine patch. Am J Psychiatry 170: 860–867.
Rose JE, Herskovic JE, Behm FM, Westman EC (2009). Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res 11: 1067–1075.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F et al (2006). An automated method for the extraction of regional data from PET images. Psychiatry Res 147: 79–89.
Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S et al (2012). Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32: 127–136.
St Helen G, Novalen M, Heitjan DF, Dempsey D, Jacob P 3rd, Aziziyeh A et al (2012). Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev 21: 1105–1114.
Strafella AP, Ko JH, Monchi O (2006). Therapeutic application of transcranial magnetic stimulation in Parkinson's disease: the contribution of expectation. Neuroimage 31: 1666–1672.
Strafella AP, Paus T, Barrett J, Dagher A (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21: RC157.
Strafella AP, Paus T, Fraraccio M, Dagher A (2003). Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126: 2609–2615.
Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D et al (2011). Positive reactions to tobacco predict relapse after cessation. J Abnorm Psychol 120: 999–1005.
Takahashi H, Fujimura Y, Hayashi M, Takano H, Kato M, Okubo Y et al (2008). Enhanced dopamine release by nicotine in cigarette smokers: a double-blind, randomized, placebo-controlled pilot study. Int J Neuropsychopharmacol 11: 413–417.
Tanner JA, Novalen M, Jatlow P, Huestis MA, Murphy SE, Kaprio J et al (2015). Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: implications for clinical implementation. Cancer Epidemiol Biomarkers Prev 24: 1239–1246.
Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M et al (2011). Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 54: 264–277.
Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P et al (2006). High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59: 389–394.
Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D et al (2005). Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem 48: 4153–4160.
Acknowledgements
We thank Greg Staios and Alexandra Andric for their assistance with this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Ciano, P., Guranda, M., Lagzdins, D. et al. Varenicline-Induced Elevation of Dopamine in Smokers: A Preliminary [11C]-(+)-PHNO PET Study. Neuropsychopharmacol 41, 1513–1520 (2016). https://doi.org/10.1038/npp.2015.305
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.305
This article is cited by
-
Exploring regulation and function of dopamine D3 receptors in alcohol use disorder. A PET [11C]-(+)-PHNO study
Neuropsychopharmacology (2021)
-
12-h abstinence-induced functional connectivity density changes and craving in young smokers: a resting-state study
Brain Imaging and Behavior (2019)