Abstract
In human evolution, social group living and Pavlovian fear conditioning have evolved as adaptive mechanisms promoting survival and reproductive success. The evolutionarily conserved hypothalamic peptide oxytocin is a key modulator of human sociality, but its effects on fear conditioning are still elusive. In the present randomized controlled study involving 97 healthy male subjects, we therefore employed functional magnetic resonance imaging and simultaneous skin conductance response (SCR) measures to characterize the modulatory influence of intranasal oxytocin (24 IU) on Pavlovian fear conditioning. We found that the peptide strengthened conditioning on both the behavioral and neural levels. Specifically, subjects exhibited faster task-related responses and enhanced SCRs to fear-associated stimuli in the late phase of conditioning, which was paralleled by heightened activity in cingulate cortex subregions in the absence of changes in amygdala function. This speaks against amygdalocentric views of oxytocin having pure anxiolytic-like effects. Instead, it suggests that the peptide enables extremely rapid and flexible adaptation to fear signals in social contexts, which may confer clear evolutionary advantages but could also elevate vulnerability for the pathological sequelae of interpersonal trauma.
Similar content being viewed by others
Introduction
Humans are inherently social, suggesting that cooperative behaviors, and a brain architecture that supports them, must have clear adaptive advantages. Current concepts of human evolution posit that the major driving force for the increase in brain, and particularly neocortex, size was the development of social group living and monogamous pair bonds (Dunbar and Shultz, 2007), thus promoting offspring survival in a hostile environment, where threat was ubiquitous, primarily through natural disasters, hunting predators, and aggressive out-groups (Öhman and Mineka, 2001).
From a neurobiological perspective, a pivotal mechanism for transforming threat into adaptive behaviors is Pavlovian fear conditioning, a form of associative learning that allows to predict aversive events (Herry and Johansen, 2014). Experimentally, Pavlovian fear conditioning is established by repeatedly coupling a neutral stimulus (CS+) with an aversive stimulus (UCS), which leads to a conditioned response (CR) to the CS+ alone (Sehlmeyer et al, 2009). However, whether or not Pavlovian fear conditioning benefits from human sociality has received less attention from neurobiology.
Key among the molecular substrates of social group living and monogamous pair bonds in humans is the hypothalamic peptide oxytocin (OXT) (Scheele et al, 2012). Studies of intranasally delivered exogenous OXT (OXTIN) have shown that the peptide produces anxiolytic-like effects as a result of dampened amygdala reactivity to threat (Eckstein and Hurlemann, 2013; Domes et al, 2007, but see Lischke et al, 2012), thereby promoting in-group trust (Kosfeld et al, 2005), cooperation (De Dreu et al, 2010) and affiliative behaviors (Hurlemann and Scheele, 2015; Rilling and Young, 2014). The amygdala–an ensemble of functionally distinct nuclei located in the medial temporal lobe–is central to a distributed neural circuitry orchestrating Pavlovian fear conditioning in humans and other species, underscoring its crucial role in promoting reproductive fitness (Herry and Johansen, 2014).
In a previous study, we found that OXTIN administered following a Pavlovian fear-conditioning paradigm and prior to fear extinction facilitated fear extinction and inhibited amygdala response (Eckstein et al, 2014b). This raised the possibility of OXTIN as a potential enhancer of psychotherapy for social anxiety disorder (SAD) and posttraumatic stress disorder (PTSD) especially. Indeed, the prevailing pathomechanistic models of anxiety disorders have emphasized a key role of Pavlovian fear conditioning in the acquisition of avoidance behavior (Lissek et al, 2008; Rauch et al, 2006).
This finding of increased fear extinction, however, seems to contradict further research showing that OXTIN facilitates bodily reactions to and episodic memory for aversive events (Striepens et al, 2012), increases the perception of social stress in the absence of support (Eckstein et al, 2014a), and promotes defensive aggression towards competing out-groups (De Dreu et al, 2010). We therefore sought to answer the question whether OXT’s influence is dependent on the timing of administration. More specifically, we examined whether the seemingly contradictory findings were based on OXT’s facilitation of two separate learning processes, namely, the learning of negative and avoidance behaviors compared with positive, prosocial behaviors. According to one model of fear conditioning and extinction as being two competing processes, the stronger of the two processes overrides and thus inhibits the other (Lissek and van Meurs, 2014). Given our previous findings that OXT prior to extinction learning increased fear extinction, we hypothesized that it could also increase fear learning if given prior to conditioning.
We therefore addressed the question of OXTIN’s influence on fear learning itself. With this study, we administered OXTIN to participants prior to Pavlovian fear conditioning in a randomized, double-blind, parallel group, placebo (PLC)-controlled study with concomitant functional magnetic resonance imaging (fMRI) and psychophysiological assessments in 97 healthy men. After the intranasal administration of either OXT (24 IU) or PLC, all participants completed an fMRI fear-conditioning procedure. We predicted that OXTIN would facilitate rather than inhibit Pavlovian fear conditioning. We expected this to be evident in psychophysiology as well as in fear-related neural networks (Sehlmeyer et al, 2009). These networks include the amygdala as well as middle and anterior parts of the cingulate cortex, which are involved in negative affect (Vogt, 2005) and whose activations have been found to be susceptible to OXTIN (Eckstein et al, 2014a, b; Preckel et al, 2014; Scheele et al, 2014a, b).
Materials and methods
Participants
Ninety-seven healthy, right-handed adult males (mean age±SD, 24.45±4.02 years) participated after giving written, informed consent. The study was approved by the local Institutional Review Board (IRB) (Identifier 329/12) and carried out in compliance with the latest revision of the Declaration of Helsinki. The study was registered in the ClinicalTrials.gov database (Identifier: NCT02156661) provided by the US National Institutes of Health. Subjects were free of current and past physical or psychiatric illness, as assessed by medical history and the Mini-International Neuropsychiatric Interview (Sheehan et al, 1997). All were non-smokers, naive to prescription-strength psychoactive medication, and had not taken any over-the-counter psychoactive medication in the past 4 weeks.
Experimental Design
We applied a randomized, placebo-controlled, double-blind, between-subject design. We preferred a parallel group design over a cross-over, within-subject design in order to avoid potentially confounding effects of repetitive fear conditioning. Volunteers were randomly assigned to intranasal administration of either OXT (24 IU; Syntocinon-Spray, Novartis; three puffs per nostril, each with 4 IU OXT) or PLC (0.9% sodium chloride solution) in accordance with current guidelines (Guastella et al, 2013). The screening of the subjects was conducted prior to the test sessions. Participants completed a baseline skin conductance response (SCR) measurement and a comprehensive neuropsychological test battery to control for possible pretreatment differences. The experimental groups did not differ in demographic variables or neuropsychological performance (cf. Table 1).
FMRI Conditioning Paradigm
We used an adapted version of a validated fMRI fear-conditioning procedure (Becker et al, 2013). During this paradigm, neutral conditioned stimuli (CS+) were paired with an aversive, unconditioned stimulus (UCS) (electric shock) in 70% contingency, while other neutral stimuli were never paired with the UCS (CS–). To account for previous findings suggesting that OXT specifically modulates processing of social stimuli (Hurlemann et al, 2010; Scheele et al, 2012), we included a social CS pair (face CS+, face CS−) and a non-social CS pair (house CS+, house CS−).
The fMRI conditioning procedure started 30 min after inhalation of the nasal spray. All CS+ and CS− stimuli were presented 30 times in total for 4000 ms each in a randomized order throughout the conditioning procedure (restriction: there were no more than two consecutive presentations of any one type of CS). CSs were separated by a variable interstimulus interval ranging from 8 to 11 s, during which subjects viewed a central fixation cross (low-level baseline).
Processing of Psychophysiological Data
Details on the psychophysiological measurement and electrical stimulation can be found in the Supplementary Material. In line with previous studies, the SCR was defined as the maximum amplitude of the eletrodermal signals during the 5 s following CS onset minus the mean electrodermal amplitude 1 s prior to stimulus onset (Becker et al, 2013). Quality checks were conducted in order to identify and exclude non-responders and invalid recordings (Eckstein et al, 2014b). We focused our SCR analysis on the data of subjects who displayed successful conditioning manifest in a larger SCR to the CS+ than to the CS− in the late conditioning phase. Of note, the treatment groups did not differ in the number of exclusions (χ2(1)=1.33, P=0.25) and 47 subjects (n=17 for OXT and n=30 for PLC) entered the final analysis.
Furthermore, to account for inter-individual differences in physiological reactivity (LaBar et al, 1998), SCR data from the fMRI session was baseline-corrected to the mean SCR in the screening session. Furthermore, consistent with previous studies (Büchel et al, 1998), the raw SCR to the electric shock (UCS) was z-standardized relative to the subject’s individual mean and SD as this SCR had no comparable counterpart in the screening session.
Acquisition and Analysis of fMRI Data
The MRI data was collected using a 1.5-T Siemens Avanto MRI system (Siemens, Erlangen, Germany). T2*-weighted echoplanar (EPI) images with blood-oxygen-level-dependent (BOLD) contrast were obtained (TR=3000 ms, TE=35 ms, matrix size: 64 × 64, pixel size: 3 × 3 × 3 mm3, slice thickness=3.0 mm, distance factor=10%, FoV=192, flip angle=90°, 36 axial slices). High-resolution anatomical images were acquired using a T1-weighted 3D MPRAGE sequence (imaging parameters: TR=1570 ms, TE=3.42 ms, matrix size: 256 × 256, pixel size: 1 × 1 × 1 mm3, slice thickness=1.0 mm, FoV=256, flip angle=15°, 160 sagittal slices).
A two-level random effects approach based on the general linear model as implemented in SPM8 was used for statistical analyses. On the first level, the four CS stimuli and the UCS were modeled as separate regressors. To account for the gradual development and temporal modulation of fear acquisition, condition-specific regressors were defined for the early (spanning the trials 1–15) and late (spanning the trials 16–30) phases of conditioning (face CS+early, face CS−early, house CS+early, house CS−early, face CS+late, face CS−late, house CS+late, house CS−late) (LaBar et al, 1998). Additional regressors for movement were included, and all regressors were convolved with the hemodynamic response function. Each experimental condition was compared relative to the low-level baseline, and differences between the CS+ and CS− conditions were computed separately for the OXT and PLC group.
Based on the first-level contrast (CS+>CS−), time-specific effects of OXT on conditioning were assessed at the second-level using repeated-measures analyses of variance (ANOVAs) with the within-subject factor ‘phase’ (early, late) and the between-subject factor ‘treatment’ (OXT, PLC). To account for an overestimation of between-subject effects in the mixed-effect models, as currently implemented in SPM, main effects of treatment were assessed using two-sample t-tests and appropriate first-level contrasts.
Furthermore, the modulatory influence of the social context on treatment effects was explored in a separate repeated-measures ANOVA model incorporating the within-subject factor ‘sociality’ (face, house) and the between-subject factor ‘treatment’ (OXT, PLC). Based on previous studies investigating the neural effects of OXT (Eckstein et al, 2014a; Scheele et al, 2014a), we defined regions of interest (ROIs) for the amygdala (Hurlemann et al, 2008) and subdivisions of the cingulate cortex (ie, the anterior cingulate cortex (ACC) and the middle cingulate cortex (MCC)) (Vogt, 2005) by applying atlas-based structural ROI masks. A significance threshold of P<0.05, corrected for multiple comparisons (family-wise error (FWE)), was used. For the ROI analyses, FWE-correction was adjusted to the size of the ROI.
In order to determine the direction and specificity of OXT effects, parameter estimates were extracted from 10-mm spheres around the peak voxel of regions showing significant treatment effects using MarsBaR toolbox (see also http://marsbar.sourceforge.net/) (Brett et al, 2002). Anatomical classification was done using WFU pick atlas, automatic anatomic labeling (aal), or Talairach Daemon (TD) labels.
Results
Behavioral Results
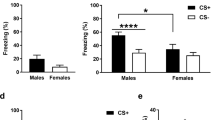
Pavlovian fear conditioning typically accelerates the reaction to the CS+ but not the CS− (Critchley et al, 2002). Therefore, mean reaction times from all participants were submitted to a repeated-measures ANOVA with the within-subject factors ‘phase’ (early: trials 1–15, late: trials 16–30), 'type' (CS+, CS−), ‘sociality’ (face, house) and ‘treatment’ (OXT, PLC) as between-subject factor. The analysis yielded a significant main effect of type (F(1,90)=7.67, P<0.01, η2=0.08) and an interaction of type and treatment (F(1,90)=3.13, P=0.04 one tailed, η2=0.03, cf. Figure 1a). Post-hoc t-tests revealed faster responses to the CS+ than to the CS− under OXTIN influence (t(32)=−3.57, P<0.01, d=−0.15) but not under PLC (P=0.45). Thus OXT seems to strengthen the conditioning effect.
Behavioral and psychophysiological effects of oxytocin on fear conditioning. Intranasal oxytocin facilitated fear conditioning, as evident in a greater reaction time difference between fear- and safety-associated stimuli (a) and enhanced electrodermal responses to the CS+ in the late conditioning phase (b). Importantly, oxytocin concurrently diminished electrodermal responses to electric shocks (c). CS+, fear-associated stimulus; CS−, safety-associated stimulus; OXT, oxytocin; PLC, placebo; SCR, skin conductance response; *P<0.05. Error bars indicate SEM.
Psychophysiological Results
We performed a repeated-measures ANOVA with the within-subject variables ‘type’ (CS+, CS−), ‘phase’ (early, late), ‘sociality’ (face, house), the between-subject factor ‘treatment’ (OXT, PLC), and the baseline-corrected SCR as dependent variable. This analysis revealed significant main effects of ‘type’ (F(1,45)=10.71, P<0.01, η2=0.19), ‘phase’ (F(1,45)=6.31, P=0.02, η2=0.12), ‘sociality’ (F(1,45)=4.89, P=0.03, η2=0.10), and ‘treatment’ (F(1,45)=8.12, P<0.01, η2=0.15), indicating successful conditioning, habituation across the phases, as well as enhanced responses to social stimuli in both groups and to all stimuli within the OXTIN group (Eckstein et al, 2014b). We also detected significant interactions of ‘type’ and ‘phase’ (F(1,45)=9.02, P<0.01, η2=0.17) and ‘sociality’ and ‘treatment’ (F(1,45)=4.91, P=0.03, η2=0.10). Differential responses to the CS+ and CS− were larger in the late phase than in the early phase of conditioning and the OXT group displayed stronger responses to social stimuli per se. Importantly, there was also a significant interaction of ‘type’, ‘phase’, and ‘treatment’ (F(1,45)=6.20, P=0.02, η2=0.12, cf. Figure 1b). Post-hoc t-tests showed that during the late phase the electrodermal response to the CS+ was significantly more pronounced under OXT (Mean±SD=1.09±0.94 mS) than under PLC (Mean±SD=0.66±0.11 mS) (t(16.27)=1.88, P=0.038, one-tailed, d=0.78), while there was no significant difference for the CS− (P=0.30).
Interestingly, the electrodermal responses to the electric shocks were decreased under OXT (0.27±0.74 z values) compared with PLC (0.74±0.69 z values) (t(95)=−3.14, P<0.01, d=0.67, cf. Figure 1c). Therefore, the OXT-induced facilitation of conditioning cannot be considered a byproduct of an enhanced UCS processing.
FMRI Results
A comparison of fear-associated stimuli and safety cues (face CS+>face CS− and house CS+>house CS−) revealed typical activations in large conditioning networks, including the insula, cingulate cortex, and prefrontal areas (cf. Table 2). A two-sample t-test yielded no general OXT effect for the processing of social vs non-social stimuli (face CS−>house CS−).
We observed a main effect of OXTIN on conditioning (CS+>CS−) such that the OXT group exhibited a significantly higher activation in the right subgenual anterior cingulate cortex (sACC) compared with the PLC group (peak MNI x, y, z=3, 29, −2, t(94)=3.57, PFWE=0.03, cf. Figure 2a and Supplementary Table S1). Thus OXT not only facilitated behavioral indices of fear conditioning but also enhanced activity in brain regions implicated in this paradigm. A repeated-measures ANOVA with the additional within-subject variable ‘phase’ (early, late) did not yield a significant interaction between phase and treatment.
Neural effects of oxytocin on fear conditioning. Intranasal oxytocin enhanced conditioning-associated activity in the subgenual anterior cingulate cortex (a) and increased neural responses in the posterior midcingulate cortex selectively in the social stimuli condition (b). In accordance with the psychophysiological observations, oxytocin dampened neural responses to the electric shock in the pregenual anterior cingulate cortex (c). OXT, oxytocin; pACC, pregenual anterior cingulate cortex; PLC, placebo; pMCC, posterior midcingulate cortex; sACC, subgenual anterior cingulate cortex; SCR, skin conductance response. **P<0.01, *P<0.05. Error bars indicate SEM.
Separate analysis for the OXT effect on fear acquisition in the social and non-social contexts revealed that OXTIN enhanced activity in the left posterior midcingulate cortex (pMCC) (peak MNI x, y, z=−15, −43, 37, t(93)=3.73, PFWE=0.04, cf. Figure 2b and Supplementary Figure S1) only for the social condition. In fact, a subsequent repeated-measures ANOVA with the within-subject variable ‘sociality’ (face, house) and the between-subject factor ‘treatment’ (OXT, PLC) showed that this OXT effect was significantly stronger for social than non-social stimuli (peak MNI x, y, z=−15, −43, 37, t(184)=3.54, PFWE=0.049).
To explore whether OXTIN influenced processing of the UCS, a whole-brain analysis was conducted. In line with our psychophysiological observations, OXT significantly decreased neural responses to the electric shock in the left pregenual ACC compared with PLC (peak MNI x, y, z=−3, 44, 4, t(94)=4.09, and −3, 59, 4, t(94)=3.62, PFWE=0.02, k=77; cf. Figure 2c). Interestingly, additional ROI analyses of the amygdala did not yield any significant results, suggesting that the observed enhancement of fear conditioning is mediated by extra-amygdalar pathways (cf. Supplementary Results).
Discussion
The rationale of the present study was to examine the modulatory effects of OXT on human fear learning using Pavlovian fear conditioning during fMRI and concomitant psychophysiological SCR measurement. Fear conditioning is an established model for mimicking the psychopathology of patients with SAD or PTSD. This preclinical study can thus contribute to closing the translational gap from animal research (Knobloch et al, 2012) to humans. Our findings indicate that OXTIN increases learning performance during conditioning as evident in both reaction times and SCRs. At the neural level, OXTIN did not affect amygdala function, suggesting that the observed behavioral and psychophysiological effects result from activity changes in extra-amygdalar regions. Indeed, we observed strengthened activity in the sACC for all fear-associated stimuli (CS+) and in the pMCC for the social fear-associated stimulus (face CS+). This specific profile suggests that OXT facilitates Pavlovian fear conditioning by potentiating neural responses to fear-associated stimuli in regions other than the amygdala, highlighting their importance in the acquisition of fear (Eckstein et al, 2014a; Striepens et al, 2012). Furthermore, in accordance with previous findings (Rash et al, 2014), OXT attenuated both neural and psychophysiological responses to the aversive electric shock. Thus an OXT-induced augmentation of fear conditioning cannot be attributed to increased pain responses but appears to be mediated by improved learning (Hurlemann et al, 2010).
The current study showed an OXTIN effect on sACC response to fear-conditioning stimuli overall. This response is not surprising owing to previous findings showing that the ACC has a crucial role within a general neural fear circuit (Hariri et al, 2003) as well as within the framework of Pavlovian fear conditioning (see reviews by Milad et al, 2007; Sehlmeyer et al, 2009). Furthermore, OXT has been found to moderate ACC activity (Gorka et al, 2015; Scheele et al, 2014a), and the ACC is characterized by a high OXT receptor density (Boccia et al, 2013). More specifically, activation in this study was found in the subgenual ACC, which is especially involved in fear responses and shows abnormal activity in mood disorders (Drevets et al, 2008). Interestingly, this neural signature of the OXTIN effect was distinct from the conditioning-related cingulate cortex responses in the PLC group, which predominantly occurred in dorsal parts. OXTIN therefore seems not only to enhance preexisting activations but also to alter the underlying neural circuitry of fear conditioning.
In contrast to the ACC, we found activation in the pMCC only in response to social stimuli following OXT administration. This could be a result of various factors. For one, OXT could be magnifying effects related to the neural integration of pain and threat (Shackman et al, 2011). In patients with SAD, which is characterized in part by an increased sensitivity to social signals, pMCC activation typically occurs in response to threat stimuli (Gaebler et al, 2014), which could suggest a similar mechanism to OXT, namely, that increased sensitivity to social stimuli takes place in the pMCC. Another reason could be the hypothesized role of this region in ‘orienting the body toward innocuous and noxious somatosensory stimuli’ (Vogt, 2005). In our study, we observed an increase in pMCC activity only under OXT and only in social stimulus-paired trials. OXT has been shown to increase social cognition in the face of both positive and negative stimuli (Shahrestani et al, 2013). It follows that OXT could generally increase the perception of social-related stimuli during fear conditioning. This could illustrate a key evolutionary function of OXT, ie, by increasing the perceived salience of social cues, OXT could optimize the efficiency of fear learning in social settings, thereby increasing the likelihood of avoiding distressing situations in interpersonal relationships. Third, it could be that social stimuli are processed via a secondary route, different from a more general processing of all stimuli in the ACC.
In our previous work, it was found that a single-dose administration of OXT potentiates episodic encoding of aversive events (Striepens et al, 2012) as well as the extinction of conditioned fear (Eckstein et al, 2014b) by amplifying activity in extra-amygdalar regions, including insular cortex, precuneus, and dorsomedial prefrontal cortex. In the present study, OXT enhanced pMCC responses to social stimuli during fear conditioning, which is consistent with findings in mice suggesting that OXT specifically facilitates social fear conditioning (Zoicas et al, 2014). Taken together with previous research (Eckstein et al, 2014a; Striepens et al, 2012), this study presents a model of OXT as an enhancer of adaptation in social contexts. This heightened ability to adapt could, however, simultaneously be the groundwork for debilitating hypersensitivity to cues if extinction is not successful. This increased vigilance or defense typically characterizes SAD (Heeren et al, 2015) and PTSD (Cantor, 2009). It is widely accepted that both disorders are based on a fear-conditioning pathomechanism. As of yet, however, there has been no data-driven explanation as to why PTSD affects victims of various types of traumas at differential rates: intentional acts of interpersonal violence, in particular sexual assault, lead to PTSD at far higher rates than do accidents or disasters (Cantor, 2009). There is evidence at least in females that interpersonal stress is linked to heightened endogenous OXT release (Taylor et al, 2010), and we have previously shown that heightened OXT increases learning in a social context (Hurlemann et al, 2010). Taken together with the results of the current study, we speculate that vulnerability for SAD and socially elicited PTSD could be associated with OXT’s enhanced adaptation to social situations as discussed above. Indeed, the rates of PTSD within a society could fit within this evolutionary model: whereas men are more likely to experience trauma overall, women experience more interpersonal violence, such as rape (Kessler et al, 1995). At the neural level, our results are consistent with findings implicating dysfunction of the ACC in the development of PTSD (Bremner et al, 2005; Hamner et al, 1999; Shin et al, 2005). Clearly, future studies are warranted to further explore possible oxytocinergic mechanisms related to SAD or PTSD.
Some research indicates that OXT could be influential in the treatment of established SAD (Labuschagne et al, 2010) and PTSD (Olff et al, 2014). Our previous findings also indicate a potential benefit via accelerated fear extinction, thus implicating OXT as a potential augmentation strategy in medication-enhanced cognitive-behavioral therapy of anxiety disorders (Eckstein et al, 2014b). The present results, however, together with findings of heightened alertness to threat (Eckstein et al, 2014a; Striepens et al, 2012) and enhanced social–emotional learning following OXT treatment (Eckstein et al, 2014b; Hurlemann et al, 2010), indicate that the peptide demands rigorous control of the therapeutic context in order to avoid suboptimal clinical outcomes. Secondary prevention intervention targeting the ‘golden hours’ (or early consolidation phase) immediately following a traumatic event might even yield contra-therapeutic effects if OXT enhances the acquisition of conditioned fear responses more efficiently than their extinction (see also Eskandarian et al, 2013).
The present study has several limitations. First, we have only tested healthy men, and given the gender-dimorphic effects of OXT and fear circuits, the extrapolation to females is hampered (Preckel et al, 2014; Scheele et al, 2014b). This is especially important as the prevalence of anxiety disorders is higher for women. This limitation, together with a general lack of sufficient literature illustrating the potential effects of OXT in PTSD and SAD, indicate that a treatment model with OXT is currently not yet feasible. Second, we did not obtain expectancy or probability ratings as subtle measures of conditioning. Third, imaging data were acquired on a 1.5-T scanner and thus the absence of an OXT effect on subcortical structures such as the amygdala might be related to the restricted spatial resolution of the scanner. Fourth, we focused our SCR analysis on the data of subjects whose SCRs indicated successful conditioning and our SCR results are therefore based on a smaller sample size than our fMRI results (cf. Supplementary Results).
In conclusion, our study presents important preliminary evidence for facilitation of fear learning by OXT. We present an evolutionary model of OXT as the basis of increased adaptation to dynamic social contexts and pose that the cost of this increase is a higher susceptibility to social trauma.
Funding and Disclosure
RH was supported by a Starting Independent Researcher Grant (‘NEMO—Neuromodulation of Emotion’) jointly provided by the Ministry of Innovation, Science, Research and Technology of the German State of North Rhine-Westphalia (MIWFT). KD was supported by the Deutsche Forschungsgemeinschaft (DFG; SFB-TRR-58 project C02). VG was supported by Chica and Heinz Schaller Research Foundation and the DFG within the Collaborative Research Center (SFB) 1134 ‘Functional Ensembles’ SFB-1134. The authors declare no conflict of interest.
References
Becker B, Androsch L, Jahn RT, Alich T, Striepens N, Markett S et al (2013). Inferior frontal gyrus preserves working memory and emotional learning under conditions of impaired noradrenergic signaling. Front Behav Neurosci 7: 197.
Boccia M, Petrusz P, Suzuki K, Marson L, Pedersen C (2013). Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 253: 155–164.
Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N et al (2005). Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 35: 791–806.
Brett M, Anton J-L, Valabregue R, Poline J-B (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage 16: S497.
Büchel C, Morris J, Dolan RJ, Friston KJ (1998). Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 20: 947–957.
Cantor C (2009). Post-traumatic stress disorder: evolutionary perspectives. Aust N Z J Psychiatry 43: 1038–1048.
Critchley HD, Mathias CJ, Dolan RJ (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653–663.
De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M et al (2010). The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328: 1408–1411.
Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 62: 1187–1190.
Drevets WC, Savitz J, Trimble M (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13: 663–681.
Dunbar RI, Shultz S (2007). Evolution in the social brain. Science 317: 1344–1347.
Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE et al (2014b). Oxytocin facilitates the extinction of conditioned fear in humans. Biol Psychiatry 78: 194–202.
Eckstein M, Hurlemann R (2013). Oxytocin—evidence for a therapeutic potential of the social neuromodulator. Der Nervenarzt 84: 1321–1328.
Eckstein M, Scheele D, Weber K, Stoffel‐Wagner B, Maier W, Hurlemann R (2014a). Oxytocin facilitates the sensation of social stress. Hum Brain Mapp 35: 4741–4750.
Eskandarian S, Vafaei AA, Vaezi GH, Taherian F, Kashefi A, Rashidy-Pour A (2013). Effects of systemic administration of oxytocin on contextual fear extinction in a rat model of post-traumatic stress disorder. Basic Clin Neurosci 4: 315–322.
Gaebler M, Daniels JK, Lamke J-P, Fydrich T, Walter H (2014). Behavioural and neural correlates of self-focused emotion regulation in social anxiety disorder. J Psychiatry Neurosci 39: 249–258.
Gorka SM, Fitzgerald DA, Labuschagne I, Hosanagar A, Wood AG, Nathan PJ et al (2015). Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology 40: 278–286.
Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM et al (2013). Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38: 612–625.
Hamner MB, Lorberbaum JP, George MS (1999). Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress Anxiety 1–14.
Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53: 494–501.
Heeren A, Mogoase C, McNally RJ, Schmitz A, Philippot P (2015). Does attention bias modification improve attentional control? A double-blind randomized experiment with individuals with social anxiety disorder. J Anxiety Disord 29: 35–42.
Herry C, Johansen JP (2014). Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci 17: 1644–1654.
Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S et al (2010). Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 30: 4999–5007.
Hurlemann R, Rehme AK, Diessel M, Kukolja J, Maier W, Walter H et al (2008). Segregating intra-amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. J Neurosci 172: 13–20.
Hurlemann R, Scheele D (2015). Dissecting the role of oxytocin in the formation and loss of social relationships. Biol Psychiatry. doi: 10.1016/j.biopsych.2015.05.013.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060.
Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH et al (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566.
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005). Oxytocin increases trust in humans. Nature 435: 673–676.
LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelp EA (1998). Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20: 937–945.
Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M et al (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35: 2403–2413.
Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M et al (2012). Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 37: 1431–1438.
Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS et al (2008). Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry 165: 124–132.
Lissek S, van Meurs B (2014). Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int J Psychophysiology. doi: 10.1016/j.ijpsycho.2014.11.006.
Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL (2007). A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 62: 1191–1194.
Öhman A, Mineka S (2001). Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev 108: 483–522.
Olff M, Koch SB, Nawijn L, Frijling JL, Van Zuiden M, Veltman DJ (2014). Social support, oxytocin and PTSD. Eur J Psychotraumatol 5: 26513.
Preckel K, Scheele D, Kendrick KM, Maier W, Hurlemann R (2014). Oxytocin facilitates social approach behavior in women. Front Behav Neurosci 8: 191.
Rash JA, Aguirre-Camacho A, Campbell TS (2014). Oxytocin and pain: a systematic review and synthesis of findings. Clin J Pain 30: 453–462.
Rauch SL, Shin LM, Phelps EA (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 60: 376–382.
Rilling JK, Young LJ (2014). The biology of mammalian parenting and its effect on offspring social development. Science 345: 771–776.
Scheele D, Kendrick KM, Khouri C, Kretzer E, Schläpfer TE, Stoffel-Wagner B et al (2014a). An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 39: 2078–2085.
Scheele D, Striepens N, Güntürkün O, Deutschländer S, Maier W, Kendrick KM et al (2012). Oxytocin modulates social distance between males and females. J Neurosci 32: 16074–16079.
Scheele D, Striepens N, Kendrick KM, Schwering C, Noelle J, Wille A et al (2014b). Opposing effects of oxytocin on moral judgment in males and females. Hum Brain Mapp 35: 6067–6076.
Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O et al (2013). Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA 110: 20308–20313.
Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V et al (2009). Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One 4: e5865.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12: 154–167.
Shahrestani S, Kemp AH, Guastella AJ (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology 38: 1929–1936.
Sheehan D, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A et al (1997). The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry 12: 232–241.
Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B et al (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62: 273–281.
Striepens N, Scheele D, Kendrick KM, Becker B, Schäfer L, Schwalba K et al (2012). Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA 109: 18144–18149.
Taylor SE, Saphire-Bernstein S, Seeman TE (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationsship? Psychol Sci 21: 3–7.
Vogt BA (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544.
Zoicas I, Slattery DA, Neumann ID (2014). Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology 39: 3027–3035.
Acknowledgements
We thank Claudia Scholz for her help with data collection and Paul Jung for providing technical support.
Author contributions
ME, DS, and RH designed the experiments; ME, AW, and KP conducted the experiments; ME, DS, BB, AW, and RH analyzed the data; and all the authors wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Eckstein, M., Scheele, D., Patin, A. et al. Oxytocin Facilitates Pavlovian Fear Learning in Males. Neuropsychopharmacol 41, 932–939 (2016). https://doi.org/10.1038/npp.2015.245
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.245
This article is cited by
-
Effects of exogenous oxytocin and estradiol on resting-state functional connectivity in women and men
Scientific Reports (2023)
-
Sniffing oxytocin: Nose to brain or nose to blood?
Molecular Psychiatry (2023)
-
Placebo and nocebo effects: from observation to harnessing and clinical application
Translational Psychiatry (2022)
-
Oxytocin-based therapies for treatment of Prader-Willi and Schaaf-Yang syndromes: evidence, disappointments, and future research strategies
Translational Psychiatry (2022)
-
Intranasal oxytocin administration impacts the acquisition and consolidation of trauma-associated memories: a double-blind randomized placebo-controlled experimental study in healthy women
Neuropsychopharmacology (2022)