Abstract
Social communication through touch and mutual grooming can convey highly salient socio-emotional signals and has been shown to involve the neuropeptide oxytocin (OXT) in several species. Less is known about the modulatory influence of OXT on the neural and emotional responses to human interpersonal touch. The present randomized placebo (PLC)-controlled within-subject pharmaco-functional magnetic resonance imaging (fMRI) study was designed to test the hypothesis that a single intranasal dose of synthetic OXT (24 IU) would facilitate both neural and emotional responses to interpersonal touch in a context- (female vs male touch) and trait- (autistic trait load) specific manner. Specifically, the experimental rationale was to manipulate the reward value of interpersonal touch independent of the intensity and type of actual cutaneous stimulation administered. Thus, 40 heterosexual males believed that they were touched by either a man or a woman, although in fact an identical pattern of touch was always given by the same female experimenter blind to condition type. Our results show that OXT increased the perceived pleasantness of female, but not male touch, and associated neural responses in insula, precuneus, orbitofrontal, and pregenual anterior cingulate cortex. Moreover, the behavioral and neural effects of OXT were negatively correlated with autistic-like traits. Taken together, this is the first study to show that the perceived hedonic value of human heterosexual interpersonal touch is facilitated by OXT in men, but that its behavioral and neural effects in this context are blunted in individuals with autistic traits.
Similar content being viewed by others
INTRODUCTION
Social communication through touch and mutual grooming is thought to have both phylogenetic and ontogenetic primacy in maintaining social cohesion and bonds in primate societies (Gallace and Spence, 2010; Hertenstein et al, 2006). The evolutionarily conserved neuropeptide oxytocin (OXT) has been shown to induce grooming and associative behaviors such as touch and huddling in rodents and monkeys (Dunbar, 2010; Winslow and Insel, 1991). However, no study has yet investigated whether it influences our subjective experience of social touch in a context-dependent manner and the neural circuits associated with it.
The neural underpinnings of human interpersonal touch are now attracting increasing interest, with recent functional magnetic resonance imaging (fMRI) studies (Gordon et al, 2013; Lindgren et al, 2012; Lovero et al, 2009; McCabe et al, 2008; Rolls et al, 2003; Voos et al, 2013) stressing the role of the pregenual anterior cingulate cortex (pACC) and orbitofrontal cortex (OFC) in coding its emotional value. Unmyelinated, C-tactile afferent fibers are sensitive to social touch (Loken et al, 2009) and project to the insula (Morrison et al, 2011), although recently the primary somatosensory cortex (SI) has also been shown to have an important role in the affective processing of touch (Gazzola et al, 2012). The SI of heterosexual men discriminates between the rewarding experience of being caressed by an attractive woman or the unpleasant feeling of being touched by another man (Gazzola et al, 2012). The hedonic value of social touch also varies substantially with personality characteristics such as autistic traits. Autism spectrum disorder (ASD) is a debilitating developmental disorder which is diagnosed on the basis of impaired social interaction and communication as well as restricted and repetitive interests and activities (American Psychiatric Association, 1994). However, a substantial majority of individuals with ASD also suffers from sensory processing deficits (Ben-Sasson et al, 2009) and a recent study found that they show a diminished response to pleasant and neutral somatosensory stimulation, and an exaggerated limbic response to unpleasant stimuli (Cascio et al, 2012). Likewise, healthy subjects with autistic traits exhibit diminished responses in the neural substrates for social touch, particularly in the OFC (Voos et al, 2013).
Largely as a result of the reported wide ranging prosocial effects of exogenous OXT administration (Eckstein and Hurlemann, 2013; Scheele et al, 2013; Striepens et al, 2011), the OXT system is considered to be a promising drug target for pharmacological treatment of the various symptoms of social impairment in ASD, including reduced interest in giving or receiving social touch. Indeed, previous animal studies have revealed that non-noxious sensory stimulation causes elevation of OXT concentrations in both blood and cerebrospinal fluid (CSF)(Uvnas-Moberg et al, 1993) and that intracerebroventricular injection of the peptide induces intense grooming in mice (Meisenberg, 1982), rats (Drago et al, 1986; Pedersen et al, 1988), and monkeys (Winslow and Insel, 1991). In humans, 15 min of massage is associated with endogenous OXT release (Morhenn et al, 2012, but see also Wikstrom et al, 2003) and experience of ‘warm touch’ between married partners enhanced salivary OXT concentrations (Holt-Lunstad et al, 2008). A recent behavioral study failed to detect an effect of OXT on touch pleasantness (Ellingsen et al, 2014), but the study did not control the sex of the caress. In the present study, we have therefore endeavored to establish directly whether intranasal administration of OXT influences responses to social touch as a function of person and context factors and which neural substrates are involved.
We used a counter-balanced double-blind, within-subject design pharmaco-fMRI approach where we twice scanned the brains of 40 heterosexual healthy male volunteers while they were exposed to various social touch conditions, and after they had received either intranasal OXT or PLC treatment. Specifically, the experimental rationale was to manipulate the reward value of interpersonal touch independent of the intensity and type of actual cutaneous stimulation administered. Thus, heterosexual males believed that they were touched by either a man or a woman although, unbeknown to the subject, an identical pattern of touch was always given by the same female experimenter. Additionally, we assessed autistic traits in our subjects by administering the autism-spectrum quotient (AQ) questionnaire (Baron-Cohen et al, 2001). We expected (Hypothesis 1) that OXT would augment the hedonic value of the more pleasant female touch and that this behavioral effect would be paralleled at the neural level by an increased response in brain areas mediating rewarding aspects of social touch, particularly the OFC and pACC. Furthermore, we predicted (Hypothesis 2) that subjects with high autistic traits would exhibit the greatest effects of the OXT treatment.
MATERIALS AND METHODS
Subjects
Forty healthy, non-smoking heterosexual male adults (mean age±SD: 25.75±3.82 years) participated in the study after giving written, informed consent. The study was approved by the institutional review board of the Medical Faculty of the University of Bonn and carried out in compliance with the latest revision of the Declaration of Helsinki. The screening of the subjects was conducted before the test sessions (see Supplementary Table S1). Subjects were free of current and past physical or psychiatric illness, as assessed by medical history and a Mini-International Neuropsychiatric Interview (Sheehan et al, 1998).
Experimental Design
In this study, we applied a randomized, placebo-controlled, double-blind, within-group design. Subjects were randomly assigned to either intranasal administration of OXT (24 IU; Syntocinon-Spray, Novartis; three puffs per nostril, each with 4 IU OXT) or PLC (sodium chloride solution) 30 min before the start of the fMRI. The intranasal administration of OXT has been shown to increase OXT concentration in the CSF (Striepens et al, 2013). Details on the tasks, fMRI procedure, and analyses can be found in the Supplementary Information.
fMRI Task
We combined and adapted two fMRI paradigms used by Gazzola et al (2012) and Kennedy et al (2009). At the start of the experiment, subjects were introduced to a male and female experimenter and informed that either the female or the male experimenter would be in the MRI room in a random order, although in fact all subjects were only touched by the female experimenter. We further told the subjects that there would be three different conditions (‘Home’, ‘Close’, and ‘Touch’). The ‘Home’ position was where the experimenter would be for the majority of time. In the ‘Home’ position, the subject was informed that the experimenter was roughly 2 m away and at a 45° angle from the junction between the MRI table and the opening of the magnet. In fact, and unbeknown to the subjects, the experimenter remained in the ‘Close’ position throughout the experiment. The ‘Close’ position was right at the junction of the MRI table and opening to the magnet. In the ‘Close’ condition, there was no physical contact between the experimenter and the subjects. In the ‘Touch’ condition, touch was administered to the shin and calf of both legs, moving from the knees toward the ankles. Before the fMRI experiment, a 20-cm zone was marked on the shin and during the 4 s of touch the complete zone was covered, resulting in a touch velocity of ∼5 cm/s. The experimenter did not know whether the female or the male experimenter condition was presented. The entire opening of the scanner was covered with a fabric so that the subjects could not see their legs or the experimenter.
In all three conditions, a photograph of the female or the male experimenter was presented and the condition (‘Home’, ‘Close’, and ‘Touch’) was relayed to the participant via text next to the photograph. In the ‘Close’ and ‘Touch’ conditions, the photographs were shown for 4 s. The duration of the ‘Home’ event was jittered between 4 and 6 s (mean: 5 s). The order of ‘Close’ and ‘Touch’ conditions was randomized, and always interleaved with the ‘Home’ condition. After each ‘Close’ and ‘Touch’ condition, the subjects rated the pleasantness of the close presence or touch by means of a visual analog scale ranging from 1 (very unpleasant; a sad smiley) to 20 (very pleasant, a happy smiley). The visual analog scale was presented for 5 s. In total, there were 20 ‘Close’, 20 ‘Touch’, and 40 ‘Home’ trials.
Acquisition and Analysis of fMRI Data
The MRI data were acquired with a 3T Siemens Trio MRI system (Siemens, Erlangen, Germany) and preprocessed and analyzed using the SPM8 software (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab 7 (The MathWorks, Natick, MA).
RESULTS
Behavioral Results
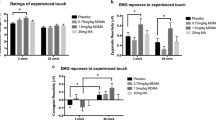
A repeated-measures analysis of variance (ANOVA) with treatment (OXT vs PLC) and sex (female vs male touch) as within-subject variables and touch pleasantness ratings as dependent variable yielded a main effect of sex (F(1,39)=64.29, P<0.01, η2=0.62) and an interaction of treatment and sex (F(1,39)=4.14, P=0.049, η2=0.10; Figure 1a), but no main effect of treatment (P=0.30). Female touch (13.83±2.53) was rated as significantly more pleasant than male touch (9.10±3.00), indicating that despite identical sensory properties our manipulation was successful in eliciting divergent hedonic values. OXT further increased the pleasantness of a caress when subjects thought it was given by a female (OXT: 14.16±2.79, PLC: 13.50±2.49, t(39)=2.77, P<0.01, d=0.25), but not when they thought it was given by a male (OXT: 8.96±3.29, PLC: 9.23±3.09, t(39)=−0.75, P=0.46, d=−0.08). However, contrary to expectation, the OXT effect on the pleasantness rating of the female caress was negatively correlated with autistic traits as measured by the AQ (r=−0.32, P=0.047; Figure 1b). This surprising result demonstrates that, in the context of social touch, healthy subjects with higher autistic traits benefited less from exogenous OXT treatment than those with better social abilities.
OXT effects on the pleasantness of touch. Intranasal administration of OXT specifically increased the pleasantness of female touch (a) and had no effect on ratings of male touch or a control condition where the female was at the same spatial distance to the subject but without any tactile contact. The behavioral OXT effect (OXT minus PLC) on female touch was more pronounced in subjects with a low autism-spectrum quotient (b). Error bars indicate the standard error of the mean (SEM). OXT, oxytocin; PLC, placebo. **P<0.01.
A repeated-measures ANOVA with the pleasantness ratings of a control condition where the female was at the same spatial distance to the subject but without any tactile contact as dependent variable also revealed a main effect of sex (F(1,39)=4.47, P=0.04, η2=0.10), with closeness of the female experimenter (12.07±3.08) being perceived as more positive than that of the male one (11.09±2.15), but there was no main or interaction effect of the treatment (all Ps>0.56). Thus, OXT specifically influenced the pleasantness ratings of female social touch but had no effect on those of close physical proximity.
fMRI Results
At the whole-brain level, under PLC treatment the caress touch stimulus, compared with the close physical proximity control condition, produced widespread activations in the social touch-processing network (Gazzola et al, 2012; Gordon et al, 2013; Lindgren et al, 2012; Lovero et al, 2009; McCabe et al, 2008; Morrison et al, 2011; Voos et al, 2013) including bilateral inferior parietal lobule, insula, somatosensory cortex, pACC, and OFC (Supplementary Tables S2 and S3). By contrast, and in line with previous research (Lovero et al, 2009), the paracentral lobule was significantly deactivated during touch. When the subjects thought that they had received female relative to male touch under PLC this elicited increased activation in the bilateral caudate (MNI coordinates x, y, z: 10, −2, 18, t(39)=4.91, and −10, −2, 16, t(39)=4.01, k=382, family-wise error corrected: PFWE=0.01) and, in accordance with a report by Gazzola et al (2012), also in the left primary SI (−36, −36, 64, t(39)=4.25, k=456, PFWE<0.01). Interestingly, a cluster in the precentral gyrus exhibited an increased activation in response to the male touch condition (−38, 20, 54, t(39)=3.80, k=303, PFWE=0.04) possibly suggesting a facilitated defensive response.
To examine specific OXT effects on female and male touch conditions, we computed the contrast (Female TouchOXT>Female TouchPLC) and (Male TouchOXT>Male TouchPLC). In the OXT session, the response to female touch was significantly enhanced in a cluster in the mid and anterior insula ranging to the OFC (40, 4, 14, t(39)=4.61 and 38, 24, 10, t(39)=4.47, k=394, PFWE=0.02; Figure 2a) and also in a cluster in the precuneus ranging to the cuneus (20, −82, 34, t(39)=4.74 and 28, −78, 28, t(39)=4.16, k=547, PFWE<0.01; Figure 2b). A ROI-based approach further revealed increased activation in the right OFC (32, 32, 2, t(39)=3.30, k=79, PFWE=0.02; Figure 2c) and pACC (−2, 38, 8, t(39)=3.05, k=105, PFWE=0.04; Figure 2d). Examination of the time courses (Figure 2a–d) illustrates the markedly different BOLD responses in the OXT and PLC sessions. For the male touch condition, only an OXT effect in the right (38, 28, −2, k=53, t(39)=4.53, PFWE<0.01) and left OFC (−32, 28, 0, k=31, t(39)=3.03, PFWE=0.04) was evident. A comparison of the OXT effects on female and male touch ((Female TouchOXT>Female TouchPLC)>(Male TouchOXT>Male TouchPLC)) showed that the OXT effects in the precuneus (24, −86, 34, t(39)=5.73 and 22, −76, 32, t(39)=4.43, k=737, PFWE<0.01) and in the pACC (−2, 38, 6, t(39)=3.24, k=79, PFWE=0.03) were significantly more pronounced in the female condition. OXT had no effect on the primary SI, even if we used Brodmann areas 1, 2, and 3 as separate ROIs with a very lenient significance threshold (P=0.005 uncorrected). Likewise, OXT did not influence neural processing during the close proximity control condition ((Female CloseOXT>Female ClosePLC) and (Male CloseOXT>Male ClosePLC), all Ps>0.05) and there was also no main treatment effect across all conditions (all Ps>0.05) demonstrating that the OXT administration did not have non-specific global effects.
OXT effects on female touch. OXT specifically enhanced neural responses to female touch in insula cortex (a), precuneus (b), orbitofrontal cortex (c), and pregenual anterior cingulate cortex (d). The shaded area represents the standard error of the mean (SEM) and the gray area indicates the duration of touch. L, left; OFC, orbitofrontal cortex; OXT, oxytocin; pACC, pregenual anterior cingulate cortex; PLC, placebo; R, right.
To determine the neural equivalent of the behavioral moderation by autistic traits, we correlated the AQ scores with the neural responses in the female ((Female TouchOXT>Baseline) and (Female TouchPLC>Baseline)) and male ((Male TouchOXT>Baseline) and (Male TouchPLC>Baseline)) touch conditions. A whole-brain analysis showed a negative association between AQ scores and the neural response to female touch under OXT in the left OFC (−30, 42, −6, t(39)=4.70, k=507, PFWE<0.01; Figure 3a). Conversely, subjects with high AQ scores demonstrated an enhanced neural response to male touch under OXT in the precentral gyrus (−30, −2, 56, t(39)=4.94, k=1009, PFWE<0.01) and also, in a ROI analysis, in the left OFC (−40, 30, −2, t(39)=2.95, k=35, PFWE=0.049; Figure 3b). There were no significant correlations under PLC. Furthermore, the AQ score was negatively associated with the OXT effect on female touch (Female TouchOXT>Female TouchPLC) in the posterior cingulate cortex (PCC) (16, −40, 30, t(39)=4.87, k=1266, PFWE<0.01) and positively with its effect on male touch in a large cluster in the medial frontal gyrus including parts of the precentral gyrus (−12, −6, 56, t(39)=5.53, k=5495, PFWE<0.01). If we confined the analysis to the predefined ROIs, then we also observed a trend for a negative moderation of the OXT effect on female touch in the left (−32, −36, 4, t(39)=2.56, k=30, PFWE=0.08) and right (32, 34, 6, t(39)=2.83, k=8, PFWE=0.06) OFC. Thus, the OXT effect on neural substrates of social touch was differentially modulated by autistic traits in the female and male touch conditions, with high AQ scores being associated with reduced responses to female touch in PCC and OFC but with increased ones to male touch in the precentral and medial frontal gyrus.
Autistic traits differentially moderate the neural response to female and male touch. Under oxytocin, neural responses to touch in the orbitofrontal cortex are negatively correlated with autistic traits in the female touch condition (a) and positively correlated in the male touch condition (b). AQ, autism-spectrum quotient; L, left; OFC, orbitofrontal cortex; OXT, oxytocin; PLC, placebo; R, right.
DISCUSSION
In the present study, we aimed at elucidating the influence of OXT on the hedonic value and neural response to social touch in men. Our findings confirmed our first hypothesis, that OXT would selectively augment subjective pleasantness ratings of female but not male touch, and that this would be due to the experience of touch and not simply as a result of close female proximity. Furthermore, OXT administration also enhanced responses to female touch in a neural circuitry involved with social touch, including insula cortex, precuneus, pACC, and OFC. However, unexpectedly we could not confirm our second hypothesis, that both the behavioral and neural OXT effects would be stronger in individuals with autistic traits. On the contrary, the opposite response pattern was observed with higher autistic traits being associated with reduced efficacy of OXT treatment, suggesting a reduced sensitivity to the peptide.
Our results strongly corroborate the notion that social touch in general and the OXT effect on hedonic value and neural response to human touch in particular are context dependent (Scheele et al, 2012; Striepens et al, 2014; Striepens et al, 2012). We have replicated previous findings (Gazzola et al, 2012) that heterosexual men perceive touch by an attractive woman as significantly more pleasant than that by a male, and that this is associated with differential responses in the primary SI, despite identical cutaneous stimulation in both cases. While OXT significantly potentiated the pleasantness ratings given in response to the female touch condition, it had no effect when subjects thought they were being touched by a male, and where they generally rated this latter experience as being mildly aversive. Elevated concentrations of endogenous OXT have been reported following both pleasant experiences such as massage or sexual intercourse (Borrow and Cameron, 2012; Morhenn et al, 2012), and unpleasant ones such as physical or psychological stress (Danevova et al, 2013; Sanders et al, 1990; Taylor et al, 2010). Obviously a system that renders aversive and potentially threatening events more pleasant would be extremely maladaptive, and therefore it is likely that the selective functional outcome of exogenous OXT treatment in relation to pleasant female touch in the current experiment reflects interactions with other neurohumoral factors. In this respect, our behavioral result is paralleled at the neural level by a greater OXT response in the pACC for female compared with male touch. Thus, in line with previous reports that the pACC encodes pleasant skin-to-skin touch (Lindgren et al, 2012; Rolls et al, 2003), the specific OXT-induced increase in pleasantness may be mediated by the pACC. Notably, the pACC has one of the highest opioid receptor binding densities in the brain (Vogt et al, 1995) and in rats OXT-enhanced grooming is attenuated by the opiate antagonist naloxone (Drago et al, 1986), suggesting that an interplay between OXT and endorphins may have contributed to the observed pattern of effects. Interestingly, a very recent human study demonstrated that increased ratings for ‘sad and rejected’ was correlated with opioidergic deactivation in the pACC, clearly evidencing this region’s relevance in the processing of social stimuli (Hsu et al, 2013).
In contrast to the pACC, the OFC exhibited an increased activation under OXT not only in the female but also in the male touch condition. OFC neurons have been shown to track salience (Ogawa et al, 2013) and an altered emotional salience of male touch does not necessarily become evident in pleasantness ratings. It thus appears that OFC activation may reflect an augmented salience of social contact, in a similar vein as it has been suggested for OXT-modulated activation in the ventral tegmental area (Groppe et al, 2013) and connectivity of the OFC (Riem et al, 2012). The concomitant OXT effect on insula and precuneus responses to female touch may also contribute to heightened subjective awareness and subsequently accentuated emotional experience of social touch. The anterior insula, the ACC, and the OFC are functionally connected (Cauda et al, 2011) and there is a striking overlap between these three regions and the proposed integral components of a theoretical framework for the bodily self (Craig, 2002) assuming that activations within them result in altered general feeling states. Similarly, the precuneus has been implicated in mediating self-consciousness and self-referential processing (Cabanis et al, 2013). Taken together, our fMRI results may therefore suggest that OXT facilitates both a self-related orientation and a greater salience of social cues which, under positive circumstances, result in a more pleasant experience of social touch.
The consequences of a more intense experience are not only moderated by the valence of the context, but also depend on pre-existing personality traits. Thus, the OXT-mediated increase in pleasantness ratings for female social touch was smaller in subjects with higher autistic traits. Aberrant social reward of touch in individuals with ASD has been associated with a diminished BOLD response to pleasant and an exaggerated limbic response to unpleasant stimuli (Cascio et al, 2012). Consistent with these results, in the present study AQ score and the OFC response to touch were negatively correlated in the more pleasant female touch condition but positively associated in the more aversive male touch one. Diminished OFC activation in response to touch in healthy adults with higher autistic traits has been reported previously (Voos et al, 2013), but we here extend this finding by showing that, under OXT, this association is reversed when the touch experienced is less pleasant. The absence of comparable associations between AQ scores and OFC activation in the PLC session in the current study may reflect both methodological and subject differences. For example, Voos et al (2013) brushed the right forearm instead of both legs, they did not instruct their subjects to rate the pleasantness of each trial in the scanner and, perhaps most importantly, more than half of their subjects were female. Despite these differences, however, the same inclination for socially less competent individuals with higher AQ scores to exhibit a diminished response to pleasant touch occurred in both studies.
Intriguingly, the context-dependent effect of touch in relation to AQ scores also emerged in other brain regions under OXT. Thus, in the female touch condition there was a negative association between the AQ scores and the OXT effect on PCC activation, whereas in the male touch condition there was a positive association with activation in the precentral gyrus. Our results thus support the idea that an enhanced PCC activation under OXT may indicate an increased processing of positive self-reflection and touch-related information (Riem et al, 2013). The PCC has been previously associated with pleasant touch (Hua et al, 2008), suggesting that a PCC-driven moderation of neural responses may contribute to the stronger behavioral effect in subjects with low AQ scores. The stronger activation of the precentral gyrus in response to the male touch condition, which was positively correlated with AQ score, may reflect the engagement of a defensive response mechanism enhanced by OXT. In monkeys, electrical stimulation of touch-sensitive neurons in the precentral gyrus elicits defensive-like movements (Graziano et al, 2002) and in humans, a tactile response inhibition task has identified this region’s involvement in generating the positive event-related potential (P300) (Huster et al, 2010), indicative of a suppressed motor reaction.
ASDs have been reported to represent the extreme end of a normal distribution of autistic-like traits, suggesting that these disorders, and autistic-like traits, are etiologically linked (Robinson et al, 2011; Lundstrom et al, 2012). In the present study, we tested healthy men with a mean AQ score of 13.20±4.64, whereas ASD patient samples typically display peak scores of 35.8±6.5 (Baron-Cohen et al, 2001). In the light of evidence indicating lower-than-normal OXT levels in ASDs (Modal et al, 1998), the observed reduction in OXT effects with higher AQ scores in the present study may suggest an ASD-symptom load-dependent dose threshold for OXT to be effective in this population. To date, evidence for an OXT dose–response relationship is still lacking (Macdonald and Feifel, 2013); assuming a linear dose–response relationship, an almost three-fold higher OXT dose would be required to reach similar effect sizes in our subjects with higher AQ scores (d=0.15) than in those with lower ones (d=0.42). For ASD patients, it may therefore be appropriate to adjust the individual OXT dose depending on the autistic symptom load. Notwithstanding, moderating effects of ASD symptoms on responsivity to OXT treatment may be domain specific.
Importantly, the absence of an OXT effect on responses to touch in the SI together with the selectivity of the OXT effects despite identical cutaneous stimulation provide strong evidence that our results cannot be attributed to peripheral changes in the sensitivity to tactile stimulation. Furthermore, OXT had no effect on mood and attention (cf. Supplementary Table S4). While we found no evidence for altered endogenous peripheral OXT levels depending on autistic traits or social touch in the PLC session (cf. Supplementary Information), we cannot rule out the possibility that autistic traits affect CSF OXT concentrations or that male and female touch have opposite effects on endogenous OXT concentrations. Clearly, further studies are warranted to disentangle the effects of OXT, endogenous opioids, and neurotransmitters such as dopamine on the multiple facets of social touch.
In conclusion, OXT augmented the experience and neural response to social touch in a context- and person-specific manner. By facilitating the hedonic value of pleasant social contact as a potential by-product of an increased self-referential processing and salience of social cues, OXT may provide the glue for the various and diverse forms of bonding that characterize us as a social species. However, OXT’s potential to alter the experience of human social touch may be diminished in individuals exhibiting low social and emotional abilities associated with autistic traits.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing: Washington, DC.
Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31: 5–17.
Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E (2009). A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J Autism Dev Disord 39: 1–11.
Borrow AP, Cameron NM (2012). The role of oxytocin in mating and pregnancy. Horm Behav 61: 266–276.
Cabanis M, Pyka M, Mehl S, Muller BW, Loos-Jankowiak S, Winterer G et al (2013). The precuneus and the insula in self-attributional processes. Cogn Affect Behav Neurosci 13: 330–345.
Cascio CJ, Moana-Filho EJ, Guest S, Nebel MB, Weisner J, Baranek GT et al (2012). Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Res 5: 231–244.
Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A (2011). Functional connectivity of the insula in the resting brain. Neuroimage 55: 8–23.
Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666.
Danevova V, Kvetnansky R, Jezova D (2013). Kinetics of oxytocin response to repeated restraint stress and/or chronic cold exposure. Horm Metab Res 45: 845–848.
Drago F, Pedersen CA, Caldwell JD, Prange AJ Jr. (1986). Oxytocin potently enhances novelty-induced grooming behavior in the rat. Brain Res 368: 287–295.
Dunbar RI (2010). The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev 34: 260–268.
Eckstein M, Hurlemann R (2013). [Oxytocin: evidence for a therapeutic potential of the social neuromodulator]. Nervenarzt 84: 1321–1328.
Ellingsen DM, Wessberg J, Chelnokova O, Olausson H, Laeng B, Leknes S (2014). In touch with your emotions: oxytocin and touch change social impressions while others' facial expressions can alter touch. Psychoneuroendocrinology 39: 11–20.
Gallace A, Spence C (2010). The science of interpersonal touch: an overview. Neurosci Biobehav Rev 34: 246–259.
Gazzola V, Spezio ML, Etzel JA, Castelli F, Adolphs R, Keysers C (2012). Primary somatosensory cortex discriminates affective significance in social touch. Proc Natl Acad Sci USA 109: E1657–E1666.
Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, Kaiser MD (2013). Brain mechanisms for processing affective touch. Hum Brain Mapp 34: 914–922.
Graziano MS, Taylor CS, Moore T (2002). Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851.
Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Grunder G et al (2013). Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry 74: 172–179.
Hertenstein MJ, Verkamp JM, Kerestes AM, Holmes RM (2006). The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genet Soc Gen Psychol Monogr 132: 5–94.
Holt-Lunstad J, Birmingham WA, Light KC (2008). Influence of a ‘warm touch’ support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med 70: 976–985.
Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H et al (2013). Response of the μ-opioid system to social rejection and acceptance. Mol Psychiatry 18: 1211–1217.
Hua QP, Zeng XZ, Liu JY, Wang JY, Guo JY, Luo F (2008). Dynamic changes in brain activations and functional connectivity during affectively different tactile stimuli. Cell Mol Neurobiol 28: 57–70.
Huster RJ, Westerhausen R, Pantev C, Konrad C (2010). The role of the cingulate cortex as neural generator of the N200 and P300 in a tactile response inhibition task. Hum Brain Mapp 31: 1260–1271.
Kennedy DP, Glascher J, Tyszka JM, Adolphs R (2009). Personal space regulation by the human amygdala. Nat Neurosci 12: 1226–1227.
Lindgren L, Westling G, Brulin C, Lehtipalo S, Andersson M, Nyberg L (2012). Pleasant human touch is represented in pregenual anterior cingulate cortex. Neuroimage 59: 3427–3432.
Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12: 547–548.
Lovero KL, Simmons AN, Aron JL, Paulus MP (2009). Anterior insular cortex anticipates impending stimulus significance. Neuroimage 45: 976–983.
Lundstrom S, Chang Z, Rastam M, Gillberg C, Larsson H, Anackarsäter H et al (2012). Autism spectrum disorders and autistic like traits: similar etiology in the extreme end and the normal variation. Arch Gen Psychiatry 69: 46–52.
Macdonald K, Feifel D (2013). Helping oxytocin deliver: considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci 7: 35.
McCabe C, Rolls ET, Bilderbeck A, McGlone F (2008). Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc Cogn Affect Neurosci 3: 97–108.
Meisenberg G (1982). Short-term behavioural effects of neurohypophyseal hormones: pharmacological characteristics. Neuropharmacology 21: 309–316.
Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C et al (1998). Plasma oxytocin levels in autistic children. Biol Psychiatry 43: 270–277.
Morhenn V, Beavin LE, Zak PJ (2012). Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Altern Ther Health Med 18: 11–18.
Morrison I, Bjornsdotter M, Olausson H (2011). Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J Neurosci 31: 9554–9562.
Ogawa M, van der Meer MA, Esber GR, Cerri DH, Stalnaker TA, Schoenbaum G (2013). Risk-responsive orbitofrontal neurons track acquired salience. Neuron 77: 251–258.
Pedersen CA, Caldwell JD, Drago F, Noonan LR, Peterson G, Hood LE et al (1988). Grooming behavioral effects of oxytocin. Pharmacology, ontogeny, and comparisons with other nonapeptides. Ann NY Acad Sci 525: 245–256.
Riem MM, van IJzendoorn MH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ (2012). No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology 37: 1257–1266.
Riem MM, van Ijzendoorn MH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ (2013). Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur Neuropsychopharmacol 23: 1288–1295.
Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happé F et al (2011). Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%). Arch Gen Psychiatry 68: 1113–1121.
Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F (2003). Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex 13: 308–317.
Sanders G, Freilicher J, Lightman SL (1990). Psychological stress of exposure to uncontrollable noise increases plasma oxytocin in high emotionality women. Psychoneuroendocrinology 15: 47–58.
Scheele D, Striepens N, Güntürkün O, Deutschlander S, Maier W, Kendrick KM et al (2012). Oxytocin modulates social distance between males and females. J Neurosci 32: 16074–16079.
Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O et al (2013). Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA 110: 20308–20313.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (Suppl 20): 22–33.
Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W et al (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 3: 3440.
Striepens N, Kendrick KM, Maier W, Hurlemann R (2011). Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Front Neuroendocrinol 32: 426–450.
Striepens N, Matusch A, Kendrick KM, Mihov Y, Elmenhorst D, Becker B et al (2014). Oxytocin enhances attractiveness of unfamiliar female faces independent of the dopamine reward system. Psychoneuroendocrinology 39: 74–87.
Striepens N, Scheele D, Kendrick KM, Becker B, Schafer L, Schwalba K et al (2012). Oxytocin facilitates protective responses to aversive social stimuli in males. Proc Natl Acad Sci USA 109: 18144–18149.
Taylor SE, Saphire-Bernstein S, Seeman TE (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci 21: 3–7.
Uvnas-Moberg K, Bruzelius G, Alster P, Lundeberg T (1993). The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand 149: 199–204.
Vogt BAW, H., Grootoonk S, Jones AKP (1995). Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from coregistered PET and MR images. Hum Brain Mapp 3: 1–12.
Voos AC, Pelphrey KA, Kaiser MD (2013). Autistic traits are associated with diminished neural response to affective touch. Soc Cogn Affect Neurosci 8: 378–386.
Wikstrom S, Gunnarsson T, Nordin C (2003). Tactile stimulus and neurohormonal response: a pilot study. Int J Neurosci 113: 787–793.
Winslow JT, Insel TR (1991). Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci 11: 2032–2038.
Acknowledgements
RH was supported by a Starting Independent Researcher Grant (‘NEMO—Neuromodulation of Emotion’) jointly provided by the Ministry of Innovation, Science, Research and Technology of the German State of North Rhine-Westphalia (MIWFT) and the University of Bonn. KMK was supported by National Natural Science Foundation of China grant (91132720).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
DS, KMK, and RH designed the experiments; DS, CK, and EK conducted the experiments; DS, KMK, CK, EK, and RH analyzed the data; BS contributed new reagents/analytic tools; DS, KMK, CK, EK, TES, BS, OG, WM, and RH wrote the paper.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Scheele, D., Kendrick, K., Khouri, C. et al. An Oxytocin-Induced Facilitation of Neural and Emotional Responses to Social Touch Correlates Inversely with Autism Traits. Neuropsychopharmacol 39, 2078–2085 (2014). https://doi.org/10.1038/npp.2014.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.78
This article is cited by
-
Neurochemical evidence for differential effects of acute and repeated oxytocin administration
Molecular Psychiatry (2021)
-
Heterosexual, gay, and lesbian people’s reactivity to virtual caresses on their embodied avatars’ taboo zones
Scientific Reports (2021)
-
Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research
Molecular Psychiatry (2021)
-
Intranasal oxytocin decreases self-oriented learning
Psychopharmacology (2021)
-
Herding in human groups is related to high autistic traits
Scientific Reports (2020)