Abstract
A recent mega-analysis combining genome-wide association study data revealed that a variant of microRNA 137 (MIR137) exhibits the most significant association with schizophrenia. Other biological evidence also consistently suggests that MIR137 may have a pivotal role in the pathogenesis of schizophrenia. However, the underlying neural mechanism remains unclear. As the disrupted dorsolateral prefrontal cortex (DLPFC) coupling with the hippocampal formation (HF) has been widely observed in schizophrenia patients, DLPFC-HF dysconnectivity can therefore be thought of as a pivotal intermediate phenotype that links genetic variants of psychiatric risk genes to schizophrenia. This study used resting-state functional magnetic resonance imaging to test whether the MIR137 variant (rs1625579) impacts DLPFC-HF functional connectivity and cognitive performance in 290 young, healthy Han Chinese individuals. To identify functional connectivity between DLPFC and HF, a seed-based functional connectivity analysis was used. The association between DLPFC-HF connectivity and working memory performance was further examined in individuals with different MIR137 genotypes. The individuals who are homozygous for the MIR137 risk allele (TT), which confers a high risk for schizophrenia, exhibited significantly different DLPFC-HF functional connectivity compared with TG individuals. Moreover, the DLPFC-HF connectivity could predict the working memory performance in MIR137 TG individuals, but not in TT individuals. The current findings obtained in a large sample of healthy participants identified potential neural mechanisms linking MIR137 with the risk of developing schizophrenia via the intermediate phenotype of DLPFC-HF connectivity.
Similar content being viewed by others
INTRODUCTION
A recent mega-analysis combining genome-wide association study data revealed that microRNA 137 (MIR137) rs1625579 exhibits the most significant association with schizophrenia, and that four predicted targets of MIR137 also display genome-wide significance (Ripke et al, 2011). Several schizophrenia-associated genes (CSMD, C10orf26, CACNA1C, TCF4, and ZNF804A) were further experimentally validated as miR-137 targets (Kim et al, 2012; Kwon et al, 2013). Furthermore, MIR137 rs1625579 were recently reported to be associated with impaired cognitive performance of schizophrenia patients (Cummings et al, 2013; Green et al, 2013). Moreover, miR-137 has a role in regulating adult neurogenesis (Szulwach et al, 2010) and neuronal maturation (Smrt et al, 2010), which is consistent with the neurodevelopmental hypothesis for the etiology of schizophrenia. All of this evidence suggests that MIR137 may have a pivotal role in the pathogenesis of schizophrenia. However, the neural mechanisms underlying this association are largely unknown.
Convergent evidence has indicated that the dorsolateral prefrontal cortex (DLPFC) is one of the most important regions involved in the pathogenesis of schizophrenia. Functional magnetic resonance imaging (fMRI) studies have demonstrated abnormal DLPFC activation in schizophrenia patients and their unaffected relatives during cognitive tasks (Callicott et al, 2003; Glahn et al, 2005; Seidman et al, 2006; Karlsgodt et al, 2007; Becker et al, 2008). More intriguingly, a disrupted DLPFC coupling with the hippocampal formation (HF) has been observed in schizophrenia patients (Meyer-Lindenberg et al, 2005), and similar findings have been reported in individuals at a high genetic risk for developing schizophrenia (Esslinger et al, 2009; Rasetti et al, 2011; Paulus et al, 2013, 2014). Previous studies have also shown that the interaction between the hippocampus and the prefrontal cortex supports working memory (WM), long-term memory, and other cognitive abilities that are abnormal in schizophrenia patients (Axmacher et al, 2008; Cohen, 2011; Hanlon et al, 2012; Supekar et al, 2013). Resting-state functional connectivity, which is measured by resting-sate fMRI, examines temporal correlations of intrinsic low-frequency fluctuations in the blood oxygenation level-dependent (BOLD) signals across brain regions, and has been proven useful for mapping brain networks (Biswal et al, 2010; van den Heuvel and Hulshoff Pol, 2010; Rosazza and Minati, 2011) and may predict individual cognitive performance (Kelly et al, 2008; Hampson et al, 2010; Supekar et al, 2013; Unschuld et al, 2013). Resting-state DLPFC-HF connectivity is therefore thought of as a pivotal intermediate phenotype linking genetic variants of psychiatric risk genes to schizophrenia and related cognitive performance.
Previous experimental evidence demonstrated that miR-137, as a brain-enriched microRNA, has important roles during both the early and late stages of adult neurogenesis, including the proliferation and differentiation of adult neural stem cells, the regulation of dendritic morphogenesis, and synaptic plasticity (Silber et al, 2008; Siegel et al, 2009; Smrt et al, 2010; Szulwach et al, 2010). Based on such biological role of miR-137, we may speculate that miR-137 influences the development and plasticity of hippocampal–cortical circuitry. Meanwhile, the dysconnectivity hypothesis of schizophrenia has been widely proven in various neuroimaging studies (Pettersson-Yeo et al, 2011). The connectivity-based neuroimaging intermediate phenotype, acting as a bridge that well links the biological function of MIR137 to the neural mechanisms of schizophrenia, may help reveal specific mechanisms that link MIR137 to the risk of schizophrenia. Therefore, by introducing the intermediate phenotype of DLPFC-HF connectivity (as measured by resting-state fMRI), we aimed to provide a novel way to understand the mechanisms of the association between MIR137 and schizophrenia.
Here, we hypothesized that individuals who are homozygous for the MIR137 risk allele (TT), which confers high risk for schizophrenia (Ripke et al, 2011), would display disrupted DLPFC-HF functional connectivity and different effects on WM performance compared with other individuals. The present study applied an imaging genetic strategy in 290 genotyped healthy Han Chinese participants to investigate the hypothesis.
MATERIALS and METHODS
Participants
We totally recruited 323 young healthy Han Chinese participants (157 males and 166 females, mean age=22.7 years, range=18–31 years, all right-handed). After they were provided with a complete description of the study, all participants provided written informed consent. The protocol was approved by the Ethics Committee of Tianjin Medical University. All of the participants were carefully screened to ensure that they had no history of neurological or psychological diseases in the subjects or their first-degree relatives, psychiatric treatment, or drug or alcohol abuse, traumatic brain injury, or visible brain lesions on conventional MRIs. All subjects were examined using the Chinese Revised Wechsler Adult Intelligence Scale (WAIS-RC) (Gong, 1982). After excluding three subjects with missing MIR137 genotype data and 30 subjects who lacked sufficient fMRI data, a total of 290 subjects were included in the data analysis of the current study.
MIR137 Genotyping
We extracted genomic DNA from whole blood using the EZgene Blood gDNA Miniprep Kit (Biomiga, San Diego, CA). MIR137 rs1625579 and ZNF804A rs1344706 were then genotyped using the iPLEX Gold Assay (Sequenom, San Diego, CA), following the manufacturer’s instructions and using primers (Supplementary Table 1), and designed using Sequenom Assay Design 3.1 software. The accuracy of genotyping was assessed by analyzing every sample in duplicate. Alleles were automatically called with Sequenom’s MassARRAY Typer 4.0 software and verified by two independent reviewers. Three subjects with missing genotype data were excluded from further analysis. MIR137 GG genotypes were not identified in our current population. The reference frequency of rs1625579 in dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=1625579) supports this phenomenon.
Image Data Acquisition
All of the subjects were scanned in the same Signa HDx 3.0 T magnetic resonance scanner (GE Healthcare, Milwaukee, WI) and were subjected to a high-resolution 3D T1-weighted brain volume (BRAVO) MRI sequence with the following parameters: repetition time (TR)=8.1 ms, echo time (TE)=3.1 ms, flip angle=13°, voxel size=1 × 1 × 1 mm3, and 176 sagittal slices. Resting-state functional imaging was subsequently performed using a single-shot-gradientecho-echo planar imaging (SS-GRE-EPI) sequence that is sensitive to BOLD contrast with the following parameters: TR=2000 ms, TE=30 ms, field of view (FOV)=240 × 240 mm2, matrix=64 × 64, flip angle=90°, voxel size=3.75 × 3.75 × 4.0 mm3, 40 slices, and 180 volumes. Before the scanning, all subjects were instructed to move as little as possible, keep their eyes closed, think of nothing in particular, and avoid falling asleep. Then, we asked subjects whether they had fallen asleep during and after the scanning to confirm that none of them had done so.
fMRI Preprocessing
We first excluded 14 subjects owing to apparent interslice motion artifacts in their raw fMRI data, which was evaluated via visual inspection by two radiologists who were blinded to genotype (C Yu and W Qin). Further data preprocessing was completed in the remaining subjects using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and in-house software. The following preprocessing steps were performed: (1) discarding of the first 10 volumes; (2) slice timing correction; (3) realigning the volumes to the first volume to correct for interscan movements; (4) spatially normalizing to a standard EPI template; (5) spatially smoothing using a 6 mm Gaussian kernel; (6) linear regression to remove the influence of head motion, whole brain signals and linear trends; (7) temporal band-pass filtration (0.01–0.08 Hz). Specifically, sixteen subjects who exhibited a maximum displacement in any of the cardinal directions (x, y, z) of >2 mm, or a maximum spin (x, y, z) of >2° were excluded from subsequent analyses.
Functional Connectivity Analyses
For each individual, we calculated voxel-wise DLPFC functional connectivity maps throughout the whole brain by taking the right DLPFC as the region-of-interest (ROI). The mask of right DLPFC was defined as a spherical region with a radius of 6 mm at the center of the MNI coordinate (42, 33, 33), according to a recent meta-analysis study (Rottschy et al, 2012). Each functional connectivity map for each individual was computed by averaging the BOLD time series in the ROI and then computing the Pearson’s correlation coefficient between the average time series and those from each voxel in the brain. The resulting correlations were transformed to approximate a Gaussian distribution using Fisher’s z transformation. Thus, the whole-brain functional connectivity map of the right DLPFC was created for each subject.
WM Performance
The WM task was performed on a computer in a quiet room outside the MRI scanner before performing magnetic resonance scanning for each subject. WM was assessed with a letter 2-back task as described previously (Ding et al, 2012). Briefly, each participant viewed a series of letters that were presented sequentially and was asked to judge whether the letter on the screen was identical to the one presented two trials back. The task consisted of three blocks (30 trials each). Before the experiment, participants were verbally instructed and had a practice block, to make sure they have understood the task before taking the formal tests. The accuracy was used as the index of WM performance in the current study.
Statistical Analysis
We used a Pearson’s χ2 test to check for gender differences and two-sample t-tests to check for differences in age, education levels, and intelligence quotient (IQ) scores between the two genotype groups. Voxel-wise one-sample t-test for the DLPFC functional connectivity map were performed in the whole group, the TT group and TG group (family-wise error (FWE) corrected p<0.05). Voxel-wise two-sample t-tests with two covariates (age and gender) were implemented in SPM8 to map group differences in the functional connectivity of the right DLPFC between MIR137 TT homozygotes and TG individuals. The FWE correction was performed using the small volume correction constrained to the predefined HF ROI (corrected P<0.05), which was created using the anatomical labels provided by the Wake Forest University PickAtlas (http://www.fmri.wfubmc.edu/downloads). The hippocampal volume was determined using Freesurfer software (Athinoula A Martinos Center for Biomedical Imaging, Charlestown, MA) (Fischl et al, 2002) and manually edited according to the T1-weighted structural MRI data. The hippocampal volume was then added as a new covariate for another round of two-sample t-tests. Fisher’s z-transformed correlation coefficients that were significantly different between two genotypes in the left HF (right DLPFC functional connectivity analyses) was used as a measure of connectivity. For each genotype group, Pearson’s correlation analysis was further performed to test the association between DLPFC-HF functional connectivity and WM performance.
Finally, to eliminate the possibility of false positives due to significantly different sample sizes in the two genotype groups, we also performed 10 000 iterations of simulation-based comparisons by randomly resampling 41 of the 249 TT subjects and comparing the random subsample with the same number of TG subjects.
RESULTS
After excluding three subjects with missing MIR137 genotype data and the 30 subjects without sufficient fMRI data from the group of 323 participants, a total of 290 subjects were finally included in the current study. The distribution of MIR137 genotypes (TT: N=249; TG: N=41; GG: N=0) did not significantly deviate from Hardy–Weinberg equilibrium (χ2=1.68, P=0.20). No significant differences in age, gender, years of education, IQ scores, and WM performance were observed between the TT and TG individuals (Table 1).
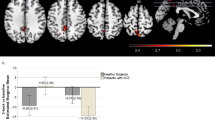
In the 290 healthy participants included in the study, we first investigated the influence of MIR137 polymorphism on the connectivity of the DLPFC to the whole brain. When the DLPFC functional connectivity map of each individual was obtained using the right DLPFC as the seed region, we performed one-sample t-test for the whole group, TT group, and TG group, and found that the three groups exhibited similar DLPFC functional connectivity patterns (Figure 1). However, individuals homozygous for the MIR137 risk allele (TT) exhibited significantly different functional connectivity between the DLPFC and the left HF (peak voxel MNI coordinate: x=−27, y=−12, z=−18; T=3.43; cluster size=51) compared with heterozygotes (TG) (Figures 2a and b). This difference survived the FWE multiple comparisons correction when the voxel-wise comparisons were corrected using the small volume correction within the predefined HF region, as applied in previous studies (Esslinger et al, 2009; Rasetti et al, 2011; Paulus et al, 2014), and remained significant after controlling for individual hippocampal volume. Interestingly, the DLPFC-HF connectivity was inversely correlated with WM performance in TG individuals, whereas such association disappeared in individuals homozygous for the MIR137 risk allele (TT) (Figure 2c). This finding demonstrated that depending on the MIR137 genotype, DLPFC-HF connectivity may affect WM performance in a totally different way.
The dorsolateral prefrontal cortex (DLPFC) functional connectivity pattern obtained by one-sample t-tests for the whole group (a), microRNA 137 (MIR137) TT homozygotes (b) and TG heterozygotes (c). Threshold was set as whole brain family-wise error (FWE) corrected P<0.05.
Effect of microRNA 137 (MIR137) rs1625579 genotype on dorsolateral prefrontal cortex-hippocampal formation (DLPFC-HF) functional connectivity and working memory performance. (a) DLPFC functional connectivity analyses using the right DLPFC (seed voxel MNI coordinate: x=42, y=33, z=33) as the seed region revealed a increased DLPFC connectivity with the left HF (peak voxel MNI coordinate: x=−27, y=−12, z=−18) in the homozygotes for the risk allele (TT) compared with TG individuals (family-wise error (FWE) corrected p<0.05). (b) Independent region-of-interest (ROI) analyses by taking the structural HF as ROI further supported that DLPFC-HF functional connectivity was different between the two different genotype groups (mean±SD). (c) Individual DLPFC-HF connectivity negatively correlated with individual working memory performance in TG individuals (right red scatter diagram), whereas the correlation did not exist in TT individuals (left blue scatter diagram).
To investigate the influence of different sample size between groups on the results, we performed simulation-based comparisons by randomly resampling 41 of the 249 TT subjects and comparing the DLPFC-HF connectivity of the random subsample with the same number of TG subjects. A total of 8879 out of the 10 000 comparisons made between groups of equal sample sizes indicated that the DLPFC-HF connectivity in the TT subsample was significantly higher than in the TG group, whereas none of the comparisons showed the inverse result (two-sample t-tests, P<0.05).
DISCUSSION
In our imaging genetic analysis, based on resting-state fMRI data obtained in a large sample of healthy Han Chinese subjects, we found that individuals homozygous for the MIR137 risk allele (TT) display significantly different DLPFC-HF functional connectivity compared with other individuals. Moreover, higher DLPFC-HF connectivity may predict worse WM performance in TG individuals; however, the prediction capability did not exist in TT individuals. These findings confirmed our hypothesis that MIR137 impacts DLPFC-HF coupling and its association with WM capacity. The current findings provide important implications regarding individual differences in the genetic contribution of MIR137 to brain function and the risk of developing schizophrenia.
One of the major findings of the present study was the influence of MIR137 on DLPFC-HF connectivity. As MIR137 rs1625579 was first reported to exhibit a strong association with schizophrenia (Ripke et al, 2011), MIR137 has been attracting increasing attention in schizophrenia research. Two studies have experimentally validated five schizophrenia-associated genes as targets of miR-137 (Kim et al, 2012; Kwon et al, 2013). Through analysis of expression profiles in normal human tissues, we found that MIR137 is specifically expressed in the brain (Liang et al, 2007) (Supplementary Figure 1). Moreover, recent studies have revealed that MIR137 rs1625579 is associated with impaired cognitive performance of schizophrenia patients (Cummings et al, 2013; Green et al, 2013), and accumulating biological evidence, including the observed role of miR-137 in regulating adult neurogenesis (Szulwach et al, 2010) and neuronal maturation (Smrt et al, 2010), supports the pivotal role of MIR137 in the pathogenesis of schizophrenia. Deciphering the neural mechanisms underlying the association between the MIR137 variant and schizophrenia may help us to establish the pathway from microRNA to risk for schizophrenia. Previous evidence has supported that miR-137 is widely expressed throughout the dentate gyrus of the hippocampus, suggesting its important functions in both developing and mature neurons (Smrt et al, 2010). Therefore, our finding of the influence of MIR137 on the DLPFC-HF connectivity supported our speculation that miR-137 affects the development and plasticity of the hippocampal–cortical circuitry. Considering that dysconnectivity of the DLPFC-HF circuit is viewed as one of the typical intermediate phenotypes in schizophrenia patients and in individuals at high genetic risk of the disease (Meyer-Lindenberg et al, 2005; Esslinger et al, 2009; Rasetti et al, 2011; Paulus et al, 2013, 2014), our findings provide a possible neural mechanism through which MIR137 may impact brain connectivity and the risk for schizophrenia. The existence of different DLPFC-HF connectivity in individuals homozygous for the MIR137 risk allele suggests that individuals who are at a high risk for schizophrenia show disrupted interactions between the DLPFC and the hippocampus. In fact, previous studies have consistently suggested that the DLPFC and the hippocampus belong to task-positive network and task-negative network, respectively (Fox et al, 2005; Hampson et al, 2010). The intrinsic functional brain organization may reflect the resting brain shifts between two different modes of processing.
Two recent studies attempted to measure the effect of MIR137 on brain structure (Lett et al, 2013) and brain function (Whalley et al, 2012). Based on neuroimaging measures of brain structure, Lett et al (2013) reported that schizophrenia patients with the MIR137 risk genotype exhibited reduced white matter integrity, as well as smaller hippocampi and larger lateral ventricles, whereas the brain structure of patients with the protective allele displayed no difference from that of healthy control subjects. Based on fMRI data during a sentence completion task in people at high genetic risk of schizophrenia or bipolar disorder, Whalley et al (2012) reported that the variant of MIR137 influences brain activation in the posterior right medial frontal gyrus. Here, we examined resting-state functional connectivity, which can be used to explore the overall organization of the functional brain network (van den Heuvel and Hulshoff Pol, 2010) and may be altered in patients with psychiatric disorders (Hulvershorn et al, 2011; Rosazza and Minati, 2011). These findings presented above suggest the putative neural mechanisms underlying the relationship between MIR137 and the risk for schizophrenia. Our results from healthy Han Chinese subjects may provide new clues for understanding the pathological mechanisms of schizophrenia.
Another interesting finding of the present study was that individual DLPFC-HF connectivity affected WM performance in a different way depending on the MIR137 genotypes. For MIR137 TG individuals who may confer lower risk for schizophrenia, individual DLPFC-HF resting-state connectivity was inversely correlated with individual WM performance. Such correlation suggests that the individuals with higher DLPFC-HF connectivity have worse WM performance. In fact, the connectivity between task-positive and -negative brain regions has been reported to be associated with WM performance (Kelly et al, 2008; Hampson et al, 2010; Unschuld et al, 2013), which is one of the most typical cognitive impairments in schizophrenia. The disappearance of the inverse relationship of connectivity and WM capacity in MIR137 high-risk individuals may suggest a disrupted modulation of DLPFC-HF connectivity with cognitive performance in TT individuals.
As the MIR137 regulating the ZNF804A (Kim et al, 2012) and ZNF804A variants has been reported to impact DLPFC-HF functional connectivity (Esslinger et al, 2009, 2011; Rasetti et al, 2011; Paulus et al, 2013), we further examined the impact of the ZNF804A SNP (rs1344706) on DLPFC-HF connectivity and also examined whether the current findings regarding MIR137 were affected by the genetic variants of ZNF804A SNP found in the present sample. All of the current results for MIR137 remain unaffected by controlling for the ZNF804A genotype as a new covariant in the group comparison analysis. Moreover, we did not find any significant differences in the resting-state DLPFC-HF connectivity or WM performance among the three ZNF804A genotypes (one-way analysis of variance, corrected P>0.05). This result may be explained by the findings of a previous study, which reported that ZNF804A influenced DLPFC-HF connectivity during the WM task but not during the resting state (Esslinger et al, 2011).
Our findings may provide new clues to understand the neural mechanisms of MIR137 on brain function and risk for schizophrenia. However, several issues should be discussed here. First, task-independent and task-dependent DLPFC-HF functional connectivity have been studied previously (Meyer-Lindenberg et al, 2005; Esslinger et al, 2009; Rasetti et al, 2011; Paulus et al, 2013, 2014), whereas different seed regions were examined. Either the DLPFC region, which shows strong activation during specific tasks, or the HF region, derived from specific brain atlases, has been selected as the seed region in these works. Our findings of the present study were based on voxel-wise DLPFC functional connectivity maps throughout the whole brain using the DLPFC as the seed region. We further investigated the influence of different ROI on the DLPFC-HF functional connectivity analyses by taking left HF as the seed region (Supplementary Text). Voxel-wise group comparisons conducted throughout the prefrontal cortex also indicated that individuals homozygous for the MIR137 risk allele (TT) display significantly different HF connectivity with the right DLPFC (peak voxel MNI coordinate: x=39, y=33, z=30; T=3.71; cluster size=43) relative to other individuals (Supplementary Figure 2). However, how to obtain the best seed region is still a question for the region-based functional connectivity analyses. Second, the difference of the sample size between two MIR137 genotypes is relatively large (TT=249 and TG=41), although simulation-based comparisons were performed in the present study by randomly resampling 41 of the 249 TT subjects and comparing the random subsample with the same number of TG subjects. The validation of our findings in independent samples is desired for the future study. Third, in addition to the disturbed DLPFC-HF connectivity, which is the focus of our current study, the functional connectivity among several other regions was also found to be different between the two groups (Supplementary Figure 3 and Supplementary Table 2). Fourth, our current analysis indicated that the DLPFC-HF connectivity was negative. Such negative functional connectivity of the DLPFC-HF was also reported in previous studies, especially for resting-state connectivity (Fox et al, 2005; Meyer-Lindenberg et al, 2005; Zhou et al, 2008; Rasetti et al, 2011). This negative connectivity might be explained as functionally competitive interactions among the neuronal activity of different brain regions (Fox et al, 2005; Zhou et al, 2008; Buckner et al, 2013). However, the sign and magnitude of functional connectivity must be interpreted cautiously because it is a relative measure that depends heavily on the preprocessing and denoizing steps of fMRI data before performing functional connectivity analysis (Buckner et al, 2013). Recently, the relationship between global signal regression during the preprocessing stage of resting-state fMRI data and negative connectivity is still under debate. Several studies have suggested that the anticorrelation is most likely caused by regressing out the global signal (Murphy et al, 2009; Weissenbacher et al, 2009; Anderson et al, 2011; Saad et al, 2012). Other studies argued that the estimates of functional connectivity by not including global effects as confounding factors might yield bias, which obscures the underlying relationship between different brain regions, and negative correlations between default mode network (task-negative network) and task-positive network could be confirmed by other methods (Chang and Glover, 2009; Carbonell et al, 2011, 2014). To test the potential influence of global signal regression on our findings, we reanalyzed our data without global signal regression. The results without global signal regression indicated that the main findings kept consistent with the results with global signal regression. There were significantly different functional connectivity of DLPFC-HF between two genotypes (P=0.041). Moreover, the DLPFC-HF connectivity without global signal regression also could predict the WM performance in MIR137 TG individuals (R=−0.437, P=0.003), but not in TT individuals (R=−0.030, P=0.322) (Supplementary Figure 4). Here we aimed to discover the group differences of the resting-state functional connectivity between different genotypes and its relationship with WM performance by considering it as a relative measure. In this opinion, the main findings with and without global signal regression kept consistent in our data. Nevertheless, although it was not the focus of our current study, we would like to note that the negative connectivity should be interpreted cautiously since we have observed the influence of global signal regression on the sign of resting-state functional connectivity. Finally, although neuroimaging evidence can increase our understanding of the association between MIR137 and schizophrenia, the effects of MIR137 rs1625579 on the expression level and the molecular function of its target downstream genes, as well as the consequent changes in the neural system function, remain unclear. Only one recent study (Guella et al, 2013) based on findings obtained through post-mortem examination has reported that miR-137 expression levels in the DLPFC are decreased in homozygous MIR137 rs1625579 TT subjects compared with TG/GG subjects. However, additional biological evidence is needed to establish a causal relationship between MIR137 rs1625579 and the risk of schizophrenia.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D (2011). Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp 32: 919–934.
Axmacher N, Schmitz DP, Wagner T, Elger CE, Fell J (2008). Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial EEG and functional magnetic resonance imaging study. J Neurosci 28: 7304–7312.
Becker TM, Kerns JG, Macdonald AW 3rd, Carter CS (2008). Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a Stroop task. Neuropsychopharmacology 33: 2619–2625.
Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM et al (2010). Toward discovery science of human brain function. Proc Natl Acad Sci USA 107: 4734–4739.
Buckner RL, Krienen FM, Yeo BT (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16: 832–837.
Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR (2003). Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry 160: 2209–2215.
Carbonell F, Bellec P, Shmuel A (2011). Global and system-specific resting-state fMRI fluctuations are uncorrelated: principal component analysis reveals anti-correlated networks. Brain Connect 1: 496–510.
Carbonell F, Bellec P, Shmuel A (2014). Quantification of the impact of a confounding variable on functional connectivity confirms anti-correlated networks in the resting-state. NeuroImage 86: 343–353.
Chang C, Glover GH (2009). Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage 47: 1448–1459.
Cohen MX (2011). Hippocampal–prefrontal connectivity predicts midfrontal oscillations and long-term memory performance. Curr Biol 21: 1900–1905.
Cummings E, Donohoe G, Hargreaves A, Moore S, Fahey C, Dinan TG et al (2013). Mood congruent psychotic symptoms and specific cognitive deficits in carriers of the novel schizophrenia risk variant at MIR-137. Neurosci Lett 532: 33–38.
Ding H, Qin W, Jiang T, Zhang Y, Yu C (2012). Volumetric variation in subregions of the cerebellum correlates with working memory performance. Neurosci Lett 508: 47–51.
Esslinger C, Kirsch P, Haddad L, Mier D, Sauer C, Erk S et al (2011). Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. NeuroImage 54: 2514–2523.
Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C et al (2009). Neural mechanisms of a genome-wide supported psychosis variant. Science 324: 605.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678.
Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE et al (2005). Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp 25: 60–69.
Gong YX (1982) Manual of Modified Wechsler Adult Intelligence Scale (WAIS-RC) (in Chinese). Hunan Med College: Changsha, China.
Green MJ, Cairns MJ, Wu J, Dragovic M, Jablensky A, Tooney PA et al (2013). Genome-wide supported variant MIR137 and severe negative symptoms predict membership of an impaired cognitive subtype of schizophrenia. Mol Psychiatry 18: 843.
Guella I, Sequeira A, Rollins B, Morgan L, Torri F, van Erp TG et al (2013). Analysis of miR-137 expression and rs1625579 in dorsolateral prefrontal cortex. J Psychiatr Res 47: 1215–1221.
Hampson M, Driesen N, Roth JK, Gore JC, Constable RT (2010). Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imag 28: 1051–1057.
Hanlon FM, Houck JM, Klimaj SD, Caprihan A, Mayer AR, Weisend MP et al (2012). Frontotemporal anatomical connectivity and working-relational memory performance predict everyday functioning in schizophrenia. Psychophysiology 49: 1340–1352.
Hulvershorn LA, Cullen K, Anand A (2011). Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imag Behav 5: 307–328.
Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M et al (2007). The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res 89: 191–197.
Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008). Competition between functional brain networks mediates behavioral variability. NeuroImage 39: 527–537.
Kim AH, Parker EK, Williamson V, McMichael GO, Fanous AH, Vladimirov VI (2012). Experimental validation of candidate schizophrenia gene ZNF804A as target for hsa-miR-137. Schizophr Res 141: 60–64.
Kwon E, Wang W, Tsai LH (2013). Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol Psychiatry 18: 11–12.
Lett TA, Chakavarty MM, Felsky D, Brandl EJ, Tiwari AK, Goncalves VF et al (2013). The genome-wide supported microRNA-137 variant predicts phenotypic heterogeneity within schizophrenia. Mol Psychiatry 18: 443–450.
Liang Y, Ridzon D, Wong L, Chen C (2007). Characterization of microRNA expression profiles in normal human tissues. BMC Genom 8: 166.
Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR et al (2005). Regionally specific disturbance of dorsolateral prefrontal–hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62: 379–386.
Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage 44: 893–905.
Paulus FM, Bedenbender J, Krach S, Pyka M, Krug A, Sommer J et al (2014). Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum Brain Mapp 35: 1190–1200.
Paulus FM, Krach S, Bedenbender J, Pyka M, Sommer J, Krug A et al (2013). Partial support for ZNF804A genotype-dependent alterations in prefrontal connectivity. Hum Brain Mapp 34: 304–313.
Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A (2011). Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev 35: 1110–1124.
Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR (2011). Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry 68: 1207–1217.
Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA et al (2011). Genome-wide association study identifies five new schizophrenia loci. Nat Genet 43: 969–976.
Rosazza C, Minati L (2011). Resting-state brain networks: literature review and clinical applications. Neurol Sci 32: 773–785.
Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB et al (2012). Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage 60: 830–846.
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A et al (2012). Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2: 25–32.
Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV et al (2006). Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res 85: 58–72.
Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M et al (2009). A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol 11: 705–716.
Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M et al (2008). miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 6: 14.
Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M et al (2010). MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells 28: 1060–1070.
Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-Lee M, Fuchs L et al (2013). Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci USA 110: 8230–8235.
Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L et al (2010). Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol 189: 127–141.
Unschuld PG, Buchholz AS, Varvaris M, van Zijl PC, Ross CA, Pekar JJ et al (2013). Prefrontal brain network connectivity indicates degree of both schizophrenia risk and cognitive dysfunction. Schizophr Bull (e-pub ahead of print).
van den Heuvel MP, Hulshoff Pol HE (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20: 519–534.
Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009). Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage 47: 1408–1416.
Whalley HC, Papmeyer M, Romaniuk L, Sprooten E, Johnstone EC, Hall J et al (2012). Impact of a microRNA MIR137 susceptibility variant on brain function in people at high genetic risk of schizophrenia or bipolar disorder. Neuropsychopharmacology 37: 2720–2729.
Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H et al (2008). Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res 100: 120–132.
Acknowledgements
This work was supported by the National Key Basic Research and Development Program (973) (Grant No. 2011CB707800), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB02030300), the Natural Science Foundation of China (Grant Nos. 91232718, 91132301 and 81201045), and the Beijing Nova Program (Grant No. 2010B061).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Liu, B., Zhang, X., Hou, B. et al. The Impact of MIR137 on Dorsolateral Prefrontal–Hippocampal Functional Connectivity in Healthy Subjects. Neuropsychopharmacol 39, 2153–2160 (2014). https://doi.org/10.1038/npp.2014.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.63
This article is cited by
-
A common variant of the NOTCH4 gene modulates functional connectivity of the occipital cortex and its relationship with schizotypal traits
BMC Psychiatry (2020)
-
ZNF804A rs1344706 interacts with COMT rs4680 to affect prefrontal volume in healthy adults
Brain Imaging and Behavior (2018)
-
Functional neuroimaging effects of recently discovered genetic risk loci for schizophrenia and polygenic risk profile in five RDoC subdomains
Translational Psychiatry (2017)
-
Abnormal Effective Connectivity in the Brain is Involved in Auditory Verbal Hallucinations in Schizophrenia
Neuroscience Bulletin (2017)
-
Polymorphisms in MIR137HG and microRNA-137-regulated genes influence gray matter structure in schizophrenia
Translational Psychiatry (2016)