Abstract
PPARγ is one of the three isoforms identified for the peroxisome proliferator-activated receptors (PPARs) and is the receptor for the thiazolidinedione class of anti-diabetic medications including pioglitazone. PPARγ has been long studied for its role in adipogenesis and glucose metabolism, but the discovery of the localization in ventral tegmental area (VTA) neurons opens new vistas for a potential role in the regulation of reward processing and motivated behavior in drug addiction. Here, we demonstrate that activation of PPARγ by pioglitazone reduces the motivation for heroin and attenuates its rewarding properties. These effects are associated with a marked reduction of heroin-induced increase in phosphorylation of DARPP-32 protein in the nucleus accumbens (NAc) and with a marked and selective reduction of acute heroin-induced elevation of extracellular dopamine (DA) levels in the NAc shell, as measured by in vivo microdialysis. Through ex vivo electrophysiology in acute midbrain slices, we also show that stimulation of PPARγ attenuates opioid-induced excitation of VTA DA neurons via reduction of presynaptic GABA release from the rostromedial tegmental nucleus (RMTg). Consistent with this finding, site-specific microinjection of pioglitazone into the RMTg but not into the VTA reduced heroin taking. Our data illustrate that activation of PPARγ may represent a new pharmacotherapeutic option for the treatment of opioid addiction.
Similar content being viewed by others
INTRODUCTION
Opioids are prototypical addictive drugs, associated with excessive morbidity and mortality (Nutt et al, 2010). Until the late twentieth century, heroin accounted for most of the disease burden from this drug class, but has now been surpassed by non-medical use of prescription opioids (Compton and Volkow, 2006). In the US alone, more than four million people abuse or are addicted to prescription opioids, which cause more than 12 000 overdose deaths annually, more than those from heroin and cocaine combined. Extensive evidence supports the efficacy of maintenance treatment with the long-acting agonists methadone and buprenorphine in opioid addiction (Amato et al, 2005), but the use of these medications is limited by their own abuse liability and the need for monitoring to maintain safety and prevent relapse. They are therefore of limited usefulness for the treatment of prescription opioid addiction in socially well-adjusted individuals. The opioid antagonist naltrexone does not have these limitations but its low patient compliance renders it less useful as a treatment (Minozzi et al, 2011). Hence, opioid addiction and dependence largely remains an unmet medical need.
The peroxisome proliferator-activated receptors (PPARs) are a group of nuclear receptor proteins, which serve primarily to regulate gene expression through their role as ligand-activated transcription factors (Michalik et al, 2006). Originally discovered as orphan nuclear receptors in the early 1990s, PPARs were found to be targets of a group of compounds known as peroxisome proliferators (Issemann and Green, 1990) because of their ability to induce the proliferation of cellular organelle peroxisomes; specifically through activation of PPARα (Lalwani et al, 1983). Three PPAR isoforms have been identified (alpha, delta, and gamma), with each being transcribed from different genes (Berger and Moller, 2002; Michalik et al, 2006; Wahli and Michalik, 2012; Lefterova et al, 2014). The primary function of PPARα is to act as a fatty acid sensor; it regulates lipoprotein metabolism and energy homeostasis (Berger and Moller, 2002; Michalik et al, 2006; Wahli and Michalik, 2012; Lefterova et al, 2014). Activation of this receptor by fibrates is used in therapy to reduce plasma lipoprotein levels. Recent findings have also shown that stimulation of PPARα function significantly attenuates the addictive properties of nicotine in preclinical models (Le Foll et al, 2013). The physiological role of the isoform PPARδ is still not well understood but its localization in the brain may suggest it plays a role in the regulation of neurological functions. Finally, PPARγ has long been known for its role in adipogenesis and glucose metabolism. This receptor is activated by molecules belonging to the class of thiazolidinediones such as pioglitazone and rosiglitazone, but the particular endogenous ligand for PPARγ still remains elusive. Thiazolidinediones have glucose-sensitizing properties and are used in therapy to treat type 2 diabetes and insulin resistance (Kersten et al, 2000). Recently, we have shown that the activation of PPARγ by pioglitazone attenuates alcohol drinking and relapse to alcohol seeking in the rat (Stopponi et al, 2011). Moreover, its co-administration with the classical opioid receptor antagonist naltrexone shows additive effects in preventing reinstatement of alcohol seeking in rodents (Stopponi et al, 2013). The potential role of PPARγ in the regulation of motivated behaviors and emotional control is supported by neuroanatomical data indicating relatively high levels of receptor expression in neurons of the caudate putamen, nucleus accumbens (NAc), septum, hypothalamus, and hippocampus (Moreno et al, 2004; Gofflot et al, 2007). A more recent study also showed significant PPARγ expression in the ventral tegmental area (VTA) where some co-localization with a TH-positive signal has been reported and in the arcuate nucleus and lateral hypothalamus where it co-localizes with α melanocyte-stimulating hormone, and proopiomelanocortin-positive cells (Sarruf et al, 2009). Moreover, recent work indicates that activation of PPARγ results in a marked inhibition of stress response in rodents, which further supports the role of this receptor in the modulation of emotional control.
Stemming from our initial work, in view of the anatomical localization of PPARγ and its emerging role in various psychiatric conditions, we thought it important to investigate the significance of this receptor in drug dependence further by exploring its role in the modulation of opioid reward and abuse.
Here, we first evaluated the effect of pioglitazone in rodent models of heroin self-administration under fixed and progressive ratio schedules of reinforcement. Moreover, because PPARγ is expressed in the VTA where it could potentially control dopamine (DA) neurotransmission, we sought to evaluate whether modulation of this catecholaminergic system might be involved in pioglitazone effects. For this purpose, we investigated the ability of this PPARγ agonist to block heroin-induced phosphorylation of DARPP-32, a marker of DA activity in the ventral striatum and in the neocortex (Svenningsson et al, 2005). In addition, using in vivo microdialysis technique, we evaluated the effect of pioglitazone on DA release in the NAc shell induced by acute heroin administration. Finally, using whole-cell patch-clamp recordings, we explored the possible mechanism of action of pioglitazone by studying its ability to modulate opioid-induced activation of VTA DA transmission. For this purpose, we explored the effect of pioglitazone on the rostromedial tegmental nucleus (RMTg), which corresponds to the posterior portion of the VTA, where opioid receptors are most abundantly expressed, and control mesolimbic DA transmission (Bourdy and Barrot, 2012).
MATERIALS AND METHODS
Detailed methods are provided in Supplementary 1.
Animals and Surgeries
Male Wistar rats and mice with neuron-specific PPARγ deletion using nestin cre-LoxP technology were employed for the studies.
For the intravenous self-administration studies, animals were prepared with intravenous catheters in the right jugular vein. For the intracranial surgery, the animals underwent stereotaxic surgery in which bilateral cannulae were implanted and aimed at the RMTg or at the VTA (for co-ordinates see Supplementary Information).
Drugs
Heroin and morphine were dissolved in sterile saline solution (0.9% NaCl) Pioglitazone (per OS administration) was prepared from Actos (30 mg) tablets. Pioglitazone (intracranial injections, Molcan Corp., Canada) and GW 9662 (Sigma-Aldrich, Italy) were dissolved as described in the Supplementary Materials.
Immunohistochemistry
pDARPP-32 staining was adapted from a previous published studies (Randall et al, 2012; Segovia et al, 2012). In brief, free-floating sections were incubated with a primary rabbit anti-pDARPP32(Thr34) (1 : 1000, sc-21601-R, Santa Cruz Biotechnology, CA, USA). Anti-rabbit HRP-conjugated envision plus (DAKO, Carpinteria, CA, USA) was used as a secondary antibody.
Pictures were taken on a Leica light microscope DM6000CS (Leica Microsystem Inc., Bannockburn, IL, USA) at × 40 magnification and BioQuant imaging software (R&M Biometrics, Nashville, TN) was used for cell density analysis. Two coronal sections (two levels) containing the PFC and NAc, both left and right side, were analyzed for pDARPP-32-positive cells (coordinates: mPFC—Bregma: +2.70 to +3.70; NAcC and NAcSh—Bregma: +0.70 to 0.70 mm (Paxinos and Watson, 2005). Results are depicted as the positively stained cells per square mm.
Microdialysis
Dialysate samples (10 μl) were injected into an HPLC equipped with a reverse phase column (C8 3.5 um, Waters, Milford, MA, USA) and a coulometric detector (ESA, Coulochem II, Bedford, MA) to quantify DA.
Electrophysiology
Neurons were visualized using an upright microscope with infrared illumination (Axioskop FS 2 plus, Zeiss), and whole-cell patch-clamp recordings were performed by using an Axopatch 200B amplifier (Molecular Devices, CA). DA neurons were identified based on previously described methods (Melis et al, 2013).
Statistical Analysis
Behavioral experiments. All data were analyzed by one- or two-way analysis of variance (ANOVA) or t-test using the STATISTICA 7 software. The significance level was established at p<0.05.
Immunohistochemistry. Data for pDARPP-32-positive cells are reported as mean values±standard error. The data from each experiment were analyzed by a one-way ANOVA with a Newman–Keuls post hoc test for pairwise comparisons. For all statistical analysis, differences between control and experimental groups were considered significant if p<0.05.
Microdialysis. ANOVA for repeated measures were applied to the raw data obtained from the serial assays of DA after each treatment. Results from treatments showing significant over-all changes were subjected to post hoc Tukey test; the significance level was established at p<0.05. The means of three consecutive samples differing by no more than 10% were considered as basal values.
Electrophysiology. Drug-induced changes in firing rate were calculated by averaging the effects after drug administration (3 min) and normalizing to the pre-drug baseline. All the numerical data are given as mean±SEM Data were compared and analyzed by utilizing two-way ANOVA for repeated measures (treatment × time), or one-way ANOVA or Student’s t-test for repeated measures, when appropriate. Statistical analysis was performed by means of the NCSS program. The significance level was established at p<0.05.
RESULTS
Pioglitazone Attenuates Operant Heroin Self-Administration under Fixed Ratio and PR Contingencies
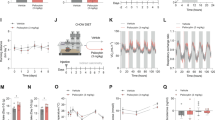
We first studied the effects of pioglitazone on intravenous heroin self-administration by training rats to lever press for heroin (20 μg/0.1 ml infusion) under a Fixed Ratio 1 schedule of reinforcement. Training continued until rats exhibited a stable level of responding. Rats were then divided into three groups and received pioglitazone (0, 30, and 60 mg/kg by gavage) in a between-subject design. Treatment was continued for five consecutive days; drug or vehicle were administered twice daily at 12 h and at 1 h before the daily 2 h heroin self-administration sessions. As shown in Figure 1a, pioglitazone (60 mg/kg) markedly reduced heroin self-administration throughout the treatment period (F(2,21)=4.34; p<0.05). Responses at the inactive control lever were not affected by the treatment (F(2,21)=0.64; p=NS). Newman–Keuls post hoc tests showed a significant effect of pioglitazone (60 mg/kg) from the first day of treatment. In a new group of rats, we then tested pioglitazone on heroin operant responding under a Progressive Ratio (PR) schedule of reinforcement where the response requirements necessary to receive a single reinforcement increased according to the following equation: [5e(injection numbers × 0.2)]−5. This resulted in the following progression of response requirements: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, etc. The breakpoint was defined as the last ratio attained by the rat prior to a 60 min period during which a ratio was not completed. Treatments and PR were continued for five consecutive days and drug (0, 30, and 60 mg/kg) was administered twice daily at 12 h and at 1 h before the PR session. As indicated in Figure 1b, pioglitazone significantly reduced the breakpoint for heroin self-administration (F (2,21)=5.39; p<0.05) at 30 (p<0.05) and 60 mg/kg (p<0.01) following Newman–Keuls post hoc analysis.
Effect of pioglitazone on operant heroin and saccharin self-administration. (a) Rats (N=8–10 per group) were treated with pioglitazone (0, 30, and 60 mg/kg, os) and tested for FR-1 heroin self-administration. Values represent the mean (±SEM) number of infusions (panel a) and responses at inactive lever (panel b). (b) Rats (N=7–9 per group) were treated with pioglitazone (0, 30, and 60 mg/kg, os) and tested for heroin self-administration under PR schedule of reinforcement. Values represent the average of BP in the 5 days of treatment. (c) Rats (N=9 per group) were treated with GW9662, pioglitazone, or their combination on FR-1 heroin self-administration. Values represent the mean (±SEM) number of infusions (panel a) and responses at inactive lever (panel b). (d) Rats (N=8 per group) were treated with pioglitazone (0 and 60 mg/kg) and tested for FR-1 saccharin self-administration. Values represent the mean (±SEM) number of saccharine rewards (panel a) and responses at inactive lever (panel b). *p<0.05, **p<0.01, ***p<0.001 compared with Pio 0.0 mg/kg. In all the other figures (a–d), significant difference from controls (0.0) *p<0.05, **p<0.005, and ***p<0.001.
Altogether, these data demonstrated that the activation of PPARγ resulted in the attenuation of the motivation to take heroin. This effect was selective because when pioglitazone (0 and 60 mg/kg) was tested against saccharin self-administration, it did not modify the animals’ operant responding (t(7)=0.87; p=NS) (Figure 1c). Moreover, at the doses tested, pioglitazone did not affect inactive lever responding (t(7)=0.64; p=NS).
The Effect of Pioglitazone on Operant Heroin Self-Administration is Blocked by Selective Antagonism at PPARγ
In another experiment, to demonstrate that the effect of pioglitazone was mediated by PPARγ, we investigated the effect of the selective PPARγ antagonist GW 9662 (0 and 5 mg/kg) on pioglitazone (0 and 60 mg/kg)-induced inhibition of Fixed Ratio 1 heroin self-administration. Rats were divided into four groups to receive pioglitazone, GW 9662, the combination of the two drugs, or both vehicles. The animals were treated twice with pioglitazone (12 and 1 h before the test) and twice with GW 9662 (30 min before each pioglitazone injection). As expected (Figure 1d), treatment with the PPARγ agonist significantly reduced operant responding for heroin (F(3,32)=5.35; p<0.01) (p<0.01 after Newman–Keuls post hoc test). This effect was prevented by pre-treatment with GW 9662. The antagonist alone did not modify heroin self-administration and inactive lever responses were not affected by the treatment (F(3,32)=0.159, p=NS).
Pioglitazone Reduces pDARPP-32 Expression Levels Induced by Heroin Self-Administration in the NAc Core and Shell
Here, we evaluated the ability of systemic pioglitazone to block DARPP-32 activation induced by heroin self-administration in key reward regions such as the NAc core and shell. The medial prefrontal cortex (mPFC) was also analyzed. DARPP-32 is a biomarker of DAergic activity as it is found in DA receptor-rich neurons and is an inhibitor of protein phosphatase 1, which modulates DAergic and glutamatergic signaling. Enhancement of DARP-32 phosporylation in the NAc reflects an increased activation of D1-receptor-mediated cellular response (Nishi et al, 1997). Rats (n=18) were trained to self-administer heroin. Training continued until rats exhibited a stable level of responding. At this point, animals received pioglitazone (0 or 60 mg/kg by gavage) for three consecutive days following the same protocol used for the self-administration studies. On the fourth day, animals received a heroin (0.4 mg/kg ip) challenge 1 h after the last pioglitazone injection. The challenge with a fixed dose of heroin was adopted as a strategy to overcome potential confounding factors related to the inhibition of heroin intake caused by pioglitazone. One additional group of rats (N=8) was naïve to heroin and served as control rats which were then transcardially perfused and brains were removed for immunohistochemistry. Five rats were excluded from the study because brains were not perfectly fixed. Heroin challenge, after a self-administration history, induced a robust increase in pDARPP-32 protein expression in the NAcSh (Figure 2a, main effect of treatment: F(2,18)=15.25, p=0.00013; Newman–Keuls post-hoc test, p<0.001) and NAcC (Figure 2b, main effect of treatment: F(2,18)=4.62, p=0.023; Newman–Keuls post-hoc test, p<0.05 compared with controls but not in the mPFC (Figure 2c, F(2,18)=1.035, p=0.3742)). This increase was eliminated by the treatment of pioglitazone (60 mg/kg) in the NAcSh (Figure 2, Newman–Keuls post-hoc test, p<0.001) and in the NAcC (Figure 2, Newman–Keuls post-hoc test, p<0.05) but not in the mPFC (Figure 2c).
Effect of heroin and pioglitazone on DARPP-32 levels in NAc shell, NAc core, and mPFC. Data are expressed as the positively stained pDARPP-32 cells per square mm in (a) NAc shell, (b) NAc core, and (c) PFC. Animals (n=18) have been trained to self-administer heroin and then divided into two groups and treated with pioglitazone or its vehicle for 3 days before to receive a heroin shot (Her SA/veh/Her shot and Her SA/Pio/Her shot). One more group of rats (n=8) with no history of heroin SA, no Pioglitazone, and no heroin shot has been used as control (veh/veh/veh). Pictures represent p-DARPP-32 immunohistochemistry following heroin self-administration. Scale bar, 100 μm.
Pioglitazone Attenuates Heroin-Induced Increase of Extracellular DA Levels in the NAc Shell
In this experiment, rats received pioglitazone (0, 30, or 60 mg/kg by gavage) for three consecutive days following the same protocol used for the self-administration studies. On the third day, a microdialysis probe was inserted into the NAc shell and core, a terminal region for mesolimbic DA fibers originating from the VTA. During the experimental session (fourth day), extracellular DA levels were evaluated under basal condition and following a heroin (0.4 mg/kg ip) challenge given 1 h after the last pioglitazone administration. Basal values of DA, expressed in fmoles/10 μl sample (mean±SEM), were: NAc shell of Veh: 37±5 (N=7), Pio 30: 40±6 (N=8), Pio 60: 36±3 (N=10); NAc core of Veh: 31±5 (N=5) Pio 30: 33±5 (N=5), Pio 60 36±4 (N=5). Rats (N=5–10 per group) were treated with pioglitazone (0, 30, and 60 mg/kg) followed by heroin (0.4 mg/kg ip) and DA dialysate in the NAc shell and core was monitored. Three-way ANOVA revealed a significant effect of brain area (F(1,34)=8.3; p<0.01), time (F(18,612)=14.17; p<0.0001) and brain area × time (F(18,612)=6.3; p<0.0001). Heroin selectively increased DA in the NAc shell. As shown in Figure 3a and b, the administration of heroin elicited a significant increase of dialysate DA in the NAc shell (max about 70% over basal) but not in the NAc core. The increase of NAc shell DA induced by heroin was significantly reduced by pioglitazone (30 or 60 mg/kg; p<0.05 after Tuckey’s post hoc test). Importantly, pioglitazone per se did not affect DA extracellular basal levels either in the NAc shell or core (Figure 1 Supplementary Material).
Effect of pioglitazone on heroin-induced DA release in the NAc shell and core DA. In vivo microdialysis: basal values of DA, expressed as fmoles/10 μl sample (a and b). Rats (N=5–10 per group) were treated with pioglitazone (0, 30, and 60 mg/kg) followed by heroin (0.4 mg/kg ip), and DA dialysate in the NAc shell and core was monitored. Results are expressed as mean±SEM of change in DA extracellular levels expressed as the percentage of basal values. The arrow indicates the administration of heroin. Solid symbols: p<0.05 vs the respective basal values; *p<0.05 vs Pio 30 group; #p<0.05 vs Pio 60 group;+p<0.05 vs Veh NAc core group (three-way ANOVA).
Stimulation of PPARγ Prevents Opioid-Induced Activation of Posterior VTA DA Neurons in Ex Vivo Electrophysiological Preparation
DA neurons recorded under current-clamp mode displayed an average frequency of 4.04±0.9 Hz (n=45) and fired spontaneously in a clock-like, single-spike mode. As shown in Figure 4a, acute bath application of morphine (0.3–3 μM, 3 min for each concentration) markedly increased the spontaneous activity of VTA DA neurons in a dose-dependent manner (F4,56=5.23, P<0.0001). Next, we examined the effect of pioglitazone (30 μM) on VTA DA neurons and then co-applied increasing concentrations (0.3–3 μM) of morphine. As shown in Figure 4b, acute application of pioglitazone (5 min) failed to prevent morphine-induced increases in firing rate (morphine 1 μM: 232.4±83.7% of basal; p=0.21, two-way ANOVA, F=1.74). However, pre-incubation of the slices with pioglitazone (30 μM) for 2 and 4 h significantly prevented morphine-induced excitation of VTA DA neurons (2 h: p=0.02, F=7.19, two-way ANOVA, n=7; 4 h: p=0.01, F=9.15, two-way ANOVA, n=8). In addition, as shown in Figure 4c, pioglitazone (3, 10, and 30 μM, 2 h) dose-dependently prevented morphine-induced excitation of DA neurons (177.3 and 125.7% of baseline at 10 and 30 μM, respectively; 10 μM: t=1.78, p=0.05, n=6; 30 mM: t=2.25, p=0.02, n=7). The concentration of morphine against which pioglitazone was tested was 1 μM, which produced about 400% increase of spontaneous firing rate of VTA DA neurons in slices from naïve rats (Figure 4a–c).
Activation of dopamine neurons by morphine is prevented by PPARγ activation ex vivo. (a) Representative traces of the spontaneous activity of a dopamine neuron during baseline (top) and morphine (1 μM, second panel), then following incubation with the PPARγ agonist pioglitazone (30 μM, 2 hs; third panel) and the subsequent morphine application (bottom panel). (b) Dose–response curves depicting averaged effects of morphine on dopamine neuron frequency of control (open circles) slices and in slice incubated for 5 min, 2 and 4 h with pioglitazone (30 μM). (c) Rate histogram depicting averaged effects of morphine (1 μM) on dopamine neuron frequency in presence of pioglitazone (3, 10, and 30 μM, 2 h); (d) Time course of the effect of GW9662 (500 nM, 2 h) alone and in combination with pioglitazone on morphine-induced excitation. (e) Time course of the effect of morphine (1 μM) in presence of pioglitazone (30 μM, 2 h) in PPARγ−/− and PPARγ+/+ mice. Data are expressed as mean±SEM.
On the basis of these results, we expected that the PPARγ antagonist GW9662 would block the actions of pioglitazone on morphine-induced excitation. As predicted, when pioglitazone was co-applied with GW9662 (500 nM, 15 min before 2 h pioglitazone incubation), morphine (1 μM) effect was fully restored (359±97.7% of basal vs control; p=0.03, t-test=2.49, n=5) (Figure 4d). Notably, GW9662 (500 nM, 2 h incubation) per se did not modify the basal spontaneous activity (t-test: p=0.29 and 0.30 vs control and GW+Pio-treated cells, respectively; n=7) nor did it modify the morphine-induced excitation of firing rate of VTA DA cells (311.8±57.1% of basal vs control: p=0.66, F1,140=0.20, two-way ANOVA; vs GW+Pio30: p=0.27, F1,126=1.36, two-way ANOVA). To confirm the lack of tonic physiological influence of PPARγ on VTA DA activity, we used neuron-specific conditional PPARγ-deficient mice generated by crossing mice carrying floxed PPARγ allele (TgH(PPARγ lox)1Mgn, TgH(PPARγ del)2Mgn) with Nestin-Cre mice (B6.Cg-Tg(Nes-cre)1Kln/J) as earlier described (Jones et al, 2002; Sarruf et al, 2009) (see also Methods section). In response to morphine (0.3–3 μM), PPARγ−/− did not differ from the PPARγ+/+ controls (Figure 2 Supplementary Material). However, pioglitazone (30 μM, 2 h incubation) was able to prevent morphine-induced excitation of VTA DA cells only in PPARγ+/+ but not in PPARγ−/− mice (228.7±16% of baseline, n=5; PPARγ−/− vs PPARγ+/+ mice: p=0.009, F1,112=11.68, two-way ANOVA; Figure 4e). This evidence suggests that genetic deletion of PPARγ in neurons led to results similar to those observed with the PPARγ antagonist GW9662.
PPARγ Agonism Attenuates Opioid-Induced Simulation of VTA DA Neurotransmission through Modulation of GABAergic Neurons of the RMTg
Consistent with the literature (Lecca et al, 2012), acute bath application of morphine at a concentration of 1 μM (for 3 min) significantly reduced IPSCs evoked by RMTg stimulation by 40.8±4.3% (n=7; F19,108=9.03; P<0.0001, Figure 5a), which was accompanied by an increased paired-pulse ratio (from IPSC2/IPSC1=1.02±0.06 to IPSC2/IPSC1=1.53±0.11; n=7, p=0.0015, paired t-test, Figure 5b). Further analysis of the CV2 and evoked IPSCs caused by morphine (Figure 4c) are indicative of a presynaptic locus for these modifications, consistent with previous reports on the effects of μ-opioid receptor stimulation of RMTg fibers (Matsui and Williams, 2011; Lecca et al, 2012). The effects of morphine were fully abolished in slices previously incubated with pioglitazone (30 μM, 2 h); hence, morphine failed to reduce gamma-aminobutyric acid (GABAA)-mediated IPSCs (106.8±16.3% of basal; n=6; F19,143=6.78; p=0.02 Figure 4d), and to modify the paired-pulse ratio (from IPSC2/IPSC1=1.3±0.4 to IPSC2/IPSC1=1.1±0.31; n=6, p=0.28, paired t-test).
Pioglitazone prevents morphine effects on RMTg-induced inhibition of VTA DA neurons. (a) Acute bath application of morphine at a concentration of 1 μM (3 min) significantly reduced IPSCs evoked by RMTg stimulation by 40.8±4.3%. (b) Increased paired-pulse ratio (from IPSC2/IPSC1=1.02±0.06 to IPSC2/IPSC1=1.53±0.11) following acute morphine application; (c) The effects of morphine was fully abolished when the slices were incubated with pioglitazone (30 μM, 2 h); hence, morphine failed to reduce GABAA-IPSCs (106.8±16.3% of basal). (d) Morphine failed to modify the paired-pulse ratio (from IPSC2/IPSC1=1.3±0.4 to IPSC2/IPSC1=1.1±0.3) in slices pre-incubated with pioglitazone.
PPARγ are Expressed in the GABAergic Region of the VTA
As already reported (Sarruf et al, 2009), PPARγ-specific staining was observed in numerous and spared cells of several areas of both rat and mouse brain. PPARγ-immunopositive reaction was detected throughout (from rostral to tail) the VTA (Figure 3a, Supplementary Material). Consistent with the notion that PPARγ is a transcription factor, PPARγ immunoreactivity was detected into cell nuclei (Figure 3b, Supplementary Material) that, however, showed different size, shape, and intensity of staining. At immunohistochemistry, PPARγ-positive cell nuclei were not observed in the brain of PPARγ knockout mice (Figure 3c, Supplementary Material). In the VTA (Figure 4, Supplementary Material), PPARγ immunoreactivity was preferentially observed in the GABAergic region of this structure including the RMTg; however, double labeling experiments revealed that these PPARγ-positive cells were not GAD67-positive GABAergic neurons. The scattered distribution of PPARγ-positive cells, among GABA-positive cell bodies and fibers suggest the possibility of a paracrine regulation of GABA neurotransmission by PPARγ.
Pioglitazone Attenuates Operant Heroin Self-Administration following Site-Specific Microinjection into the RMTg, but not into the VTA
To confirm the mechanism of action, rats trained to self-administer heroin on an Fixed Ratio 1 schedule were bilaterally implanted with intracranial cannulas for site-specific injection into RMTg or into the VTA. In a within-subject design experiment, rats received pioglitazone (0 and 5 μg/0.6 μl) 12 and 1 h before the test sessions. As shown in Figure 6a, b, rats treated with pioglitazone into the RMTg significantly reduced their intake of heroin compared with vehicle-treated controls (t(17)=2.495; p<0.05) whereas, as shown in Figure 5c, d, pioglitazone injected into the VTA did not show any effect (t(16)=1.146; p<NS). The inactive control lever was not affected by the treatments (t(17)=1.005; p=NS) (RMTg) and (t(16)=0.12; p=NS) (VTA). Following the experiments, histological verification revealed incorrect cannula placements in nine rats. Data from these rats were not included in the statistical analysis.
Pioglitazone attenuates operant heroin self-administration following site-specific microinjection into the RMTg, but not into the VTA. (a) Effect of intra-RMTg injections of pioglitazone (0 or 5 μg/0.6 μl) in rats (N=18) subjected to heroin FR-1 self-administration. Values represent the mean (±SEM) number of infusions (panel a) and responses at inactive lever (panel b). (b) Schematic representation showing correct (filled circles) and incorrect (unfilled triangles) cannulae placements. (c) Effect of intra-VTA injections of pioglitazone (0 or 5 μg/0.6 μl) in rats (N=17) subjected to heroin FR-1 self-administration. Values represent the mean (±SEM) number of infusions (panel a) and responses at inactive lever (panel b). (d) Schematic representation showing correct (filled circles) and incorrect (unfilled triangles) cannulae placements. Drawing is from the atlas of Paxinos and Watson. Significant difference from controls (0.0) *p<0.05.
DISCUSSION
We showed that activation of PPARγ by pioglitazone, at the dose of 60 mg/kg, markedly reduced heroin self-administration throughout the treatment period. When pioglitazone administration was discontinued, heroin self-administration returned to baseline level. We also evaluated the effect of pioglitazone on heroin operant responding under a PR schedule of reinforcement. This model is highly useful for the study of positive reinforcement and is predictive of the efficacy of medication to attenuate the reinforcing effects of drug of abuse (Katz and Goldberg, 1988). Under this operant contingency, the rats showed a high self-administration breakpoint reflecting their strong motivation to take heroin. Pioglitazone significantly reduced it at both doses tested. Importantly, pioglitazone failed to alter saccharin self-administration, indicating that its effect is specific for heroin and does not depend upon alteration of animals’ motor performances or general inhibition of motivated behavior. To confirm that the effect of pioglitazone was mediated by selective activation of PPARγ, we pretreated the rats with the selective receptor antagonist GW 9662 that, as expected, fully reversed its effects on heroin. The antagonist alone did not modify heroin self-administration.
The mesolimbic DA system that projects from the VTA to the NAc plays a major role in mediating the motivational properties of drugs of abuse (Fibiger et al, 1987; Di Chiara, 1995; Wise, 1996; Nestler, 2005; Luscher and Malenka, 2011). Substantial evidence from both human and animal studies demonstrates that abused drugs increase DA transmission in the VTA/NAc pathway (Di Chiara and Imperato, 1988; Schultz, 2002; Volkow et al, 2007). A postsynaptic marker of DAergic activity is found in DA receptor-rich neurons especially in the NAc, the caudate putamen, and the prefrontal cortex is DARPP-32 (Ouimet et al, 1984). The inhibition of protein phosphatase-1 activity that occurs following phosphorylation of DARPP-32 at Thr34 promotes the phosphorylation of downstream target proteins, thereby amplifying responses mediated by the activation of DA D1 receptors (Fienberg et al, 1998). Enhancement of DARPP-32 phosphorylation has been proposed as a common mechanism, shared by all drugs of abuse including opioids, and is supposed to reflect not only their ability to activate DAergic signaling pathways but also their addictive potential (Svenningsson et al, 2005; Zachariou et al, 2006; Mahajan et al, 2009). Moreover, it has been suggested that the inhibition of Darpp-32 signaling pathways may represent a therapeutic strategy to treat opioid addiction (Mahajan et al, 2009). On the basis of these considerations together with the recent demonstration of PPARγ expression in the VTA (Sarruf et al, 2009), we hypothesized that pioglitazone effects on heroin self-administration might have been linked to the ability of this drug to inhibit DARPP-32 phosphorylation pathways via the reduction of mesolimbic DAergic transmission. To explore this possibility, we evaluated the effect of subchronic pioglitazone on the expression of pDARPP-32 in the NAc core and shell and in the mPFC in rats subjected to the same operant heroin self-administration training described above. As expected, heroin treatment significantly enhanced pDARPP-32 levels in the NAc core and shell, whereas protein expression remained at baseline levels in the mPFC. Notably, pretreatment with pioglitazone at the same dose that reduced heroin self-administration in previous experiments completely blocked this effect of the opioid agonist both in the NAc core and shell. At this point, to confirm at mechanistic level that the effect of pioglitazone on DARPP-32 was due to its capacity to attenuate mesolimbic DA transmission, we evaluated by in vivo microdialysis whether the activation of PPARγ attenuates the ability of heroin to increase extracellular DA in the NAc. Extracellular DA levels were evaluated under basal conditions and following a challenge with the same dose of heroin (0.4 mg/kg ip) that was effective in enhancing pDARPP-32 levels in rats with a history of opioid self-administration. As non-contingent heroin administration preferentially increases dialysate DA in the NAc shell as compared with the core, the two subregions were studied separately (Tanda et al, 1997). We found that chronic treatment with pioglitazone is effective in reducing heroin-induced increase of extracellular DA in the NAc shell. Importantly, we showed that oral injection of pioglitazone, at the doses of 30 and 60 mg/kg, did not alter DA extracellular levels in the NAc shell per se. DA levels in the NAc core were neither affected by the heroin challenge nor by pioglitazone administration. In summary, these data confirm that the inhibition of mesolimbic DA transmission following pioglitazone occurs and this could be the reason why it prevented the effects of heroin on DARPP-32 phosphorylation. An important difference between immunohistochemistry and neurochemical data is that in the former, DARPP-32 activation was observed in both the NAc core and shell, whereas in the microdialysis, the increase in DA release was found only in the shell. However, as it has been previously shown, acute administration of drugs of abuse enhances extracellular DA levels only in the NAc shell, whereas following protracted exposure to drugs, the NAc core is progressively recruited and DA neurotransmission is enhanced also in this region (Lecca et al, 2007). Here, DARPP-32 expression was measured in rats with a history of heroin self-administration whereas microdialysis was carried out under acute condition in naive rats; hence, our findings are perfectly consistent with the current literature and support the role of mesolimbic neurotransmission in the regulation of pioglitazone effects on heroin.
In the VTA, μ-opioid receptors are largely expressed in presynaptic GABA neurons. The activation of opioid receptors leads to strong inhibition of GABA neurons and disinhibition of DA cells (Johnson and North, 1992; Chieng and Christie, 1994; Bonci and Williams, 1996; Jalabert et al, 2011; Lecca et al, 2012; Meye et al, 2012) and this represents the canonical mechanism through which opioids modulate mesolimbic DA transmission and reward. Thus, the question we asked next was whether pioglitazone’s effect on acute heroin administration was dependent upon a role of PPARγ in modulating DA neuronal activity in the VTA. To answer this question, we tested the effect of pioglitazone on morphine (the active metabolite of heroin in the brain)-induced stimulation of DA cell firing from acute brain slices containing the VTA by using whole-cell patch-clamp recordings. In accordance with the literature, we found that the spontaneous activity of VTA DA neurons was increased after acute bath application of morphine; this effect was prevented on pre-incubating the brain slices with pioglitazone. As we expected, the selective PPARγ antagonist, GW9662, blocked the actions of pioglitazone on morphine-induced excitation. We then asked whether PPARγ has a tonic physiological role in the regulation of VTA DA neurotransmission. To answer this question, we recorded from VTA slices of neuron-specific PPARγ KO mice. Notably, compared with wild-type controls, we did not observe differences in the morphine-induced activation of VTA DA neurotransmission in these animals. However, in PPARγ KO mice, pioglitazone failed to block morphine effect on VTA DA cells further confirming that drug effect is specifically mediated by this receptor type.
At this point, using confocal microscopy combined with TH, GAD67, and PPARγ triple immunostaining, we decided to explore at morphological level the distribution of PPARγ in the VTA. Results showed that immunopositive cell for to PPARγ were preferentially observed in the GABAergic region of the VTA where they were identified in close proximity of GABAergic terminal and cell bodies.
GABAergic afferents onto VTA DA neurons can be distinguished into two distinct subpopulations, ie, intrinsic and extrinsic to the VTA. The first consists of small interneurons forming local circuitry with DA cells (Garzon and Pickel, 2001; Margolis et al, 2012). The second is composed of extrinsic afferents among which those originating from the RMTg play a crucial role not only in controlling the firing of DA cells, but also in mediating some of the effects of drugs of abuse (Lecca et al, 2012). Both afferents have been linked to the effects of opioids on VTA DA; however, recent evidence strongly points to the extrinsic circuitry as the one playing a major role (Jalabert et al, 2011; Matsui and Williams, 2011; Lecca et al, 2012; Hjelmstad et al, 2013). RMTg GABA neurons express μ-opioid receptors whose stimulation results in a marked inhibition of GABAergic activity and subsequent disinhibition of VTA DA cell activity (Lecca et al, 2011; Matsui and Williams, 2011; Lecca et al, 2012). We therefore decided to test whether pioglitazone would affect the morphine-induced inhibition of RMTg-evoked GABAA-IPSCs recorded from VTA DA cells. The application of morphine significantly reduced IPSCs evoked by RMTg stimulation and this effect could be prevented through incubation of the slices with pioglitazone. This mechanism was then confirmed in rats trained to self-administer heroin in which operant responding was significantly attenuated by site-specific microinjection of pioglitazone into the RMTg but not into rostral portion of the VTA.
On the basis of their mechanism of actions, drugs of abuse can be divided into two major classes. On the one hand, there are the psychostimulants that enhance mesolimbic DA transmission by acting directly in the NAc where they facilitate DA release or decrease DA re-uptake. On the other hand, there are opioids, alcohol, nicotine, and to some extent cannabinoids, that by inhibiting GABAergic inhibitory transmission in the VTA-RMTg region facilitate the disinhibition of mesolimbic DA circuitry thus enhancing extracellular DA levels in the NAc (Luscher and Ungless, 2006). Considering the mechanism of action PPARγ, we speculate that the therapeutic potential of agonists like pioglitazone should be particularly important for this latter group of addictive agents.
In summary, our results demonstrate that the activation of PPARγ may represent a promising approach for the treatment of opioids dependence. At present, the most effective medications in opioid addiction are based on methadone and buprenorphine. However, the abuse liability potential of these compounds severely limits their clinical application such that in practice it is almost exclusively restricted to long-term heroin addicts. Extremely problematic remains, instead, the possibility to use these pharmacological remedies in prescription opioid or early stage heroin abusers, which represent a growing patient population. Our data indicate that centrally active PPARγ agonists hold the promise to fill this need. The PPARγ agonist pioglitazone has been in clinical use for several years in the treatment of type 2 diabetes and its tolerability has been largely demonstrated. The ability of pioglitazone to attenuate the addictive properties of heroin opens the prospect for immediate clinical investigation to determine its efficacy in humans.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP (2005). An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. J Subst Abuse Treat 28: 321–329.
Berger J, Moller DE (2002). The mechanisms of action of PPARs. Annu Rev Med 53: 409–435.
Bonci A, Williams JT (1996). A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron 16: 631–639.
Bourdy R, Barrot M (2012). A new control center for dopaminergic systems: pulling the VTA by the tail. Trends Neurosci 35: 681–690.
Chieng B, Christie MJ (1994). Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol 113: 303–309.
Compton WM, Volkow ND (2006). Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81: 103–107.
Di Chiara G (1995). The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend 38: 95–137.
Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
Fibiger HC, LePiane FG, Jakubovic A, Phillips AG (1987). The role of dopamine in intracranial self-stimulation of the ventral tegmental area. J Neurosci 7: 3888–3896.
Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A et al (1998). DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science 281: 838–842.
Garzon M, Pickel VM (2001). Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse 41: 311–328.
Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J et al (2007). Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell 131: 405–418.
Hjelmstad GO, Xia Y, Margolis EB, Fields HL (2013). Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J Neurosci 33: 6454–6459.
Issemann I, Green S (1990). Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–650.
Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M et al (2011). Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci USA 108: 16446–16450.
Johnson SW, North RA (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12: 483–488.
Jones JR, Shelton KD, Guan Y, Breyer MD, Magnuson MA (2002). Generation and functional confirmation of a conditional null PPARgamma allele in mice. Genesis 32: 134–137.
Katz JL, Goldberg SR (1988). Preclinical assessment of abuse liability of drugs. Agents Actions 23: 18–26.
Kersten S, Desvergne B, Wahli W (2000). Roles of PPARs in health and disease. Nature 405: 421–424.
Lalwani ND, Reddy MK, Qureshi SA, Sirtori CR, Abiko Y, Reddy JK (1983). Evaluation of selected hypolipidemic agents for the induction of peroxisomal enzymes and peroxisome proliferation in the rat liver. Hum Toxicol 2: 27–48.
Le Foll B, Di Ciano P, Panlilio LV, Goldberg SR, Ciccocioppo R (2013). Peroxisome proliferator-activated receptor (PPAR) agonists as promising new medications for drug addiction: preclinical evidence. Curr Drug Targets 14: 768–776.
Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G (2007). Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology 194: 103–116.
Lecca S, Melis M, Luchicchi A, Ennas MG, Castelli MP, Muntoni AL et al (2011). Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology 36: 589–602.
Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M (2012). Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology 37: 1164–1176.
Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S (2014). PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab 25: 293–302.
Luscher C, Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663.
Luscher C, Ungless MA (2006). The mechanistic classification of addictive drugs. PLoS Med 3: e437.
Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Sykes DE, Hu Z et al (2009). Therapeutic targeting of ‘DARPP-32’: a key signaling molecule in the dopiminergic pathway for the treatment of opiate addiction. Int R Neurobiol 88: 199–222.
Margolis EB, Toy B, Himmels P, Morales M, Fields HL (2012). Identification of rat ventral tegmental area GABAergic neurons. PloS One 7: e42365.
Matsui A, Williams JT (2011). Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci 31: 17729–17735.
Melis M, Scheggi S, Carta G, Madeddu C, Lecca S, Luchicchi A et al (2013). PPARalpha regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving alpha7 nicotinic acetylcholine receptors. J Neurosci 33: 6203–6211.
Meye FJ, van Zessen R, Smidt MP, Adan RA, Ramakers GM (2012). Morphine withdrawal enhances constitutive mu-opioid receptor activity in the ventral tegmental area. J Neurosci 32: 16120–16128.
Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ et al (2006). International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58: 726–741.
Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A (2011). Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 13: CD001333.
Moreno S, Farioli-Vecchioli S, Ceru MP (2004). Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience 123: 131–145.
Nestler EJ (2005). Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445–1449.
Nishi A, Snyder GL, Greengard P (1997). Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci 17: 8147–8155.
Nutt DJ, King LA, Phillips LD (2010). Drug harms in the UK: a multicriteria decision analysis. Lancet 376: 1558–1565.
Ouimet CC, Miller PE, Hemmings HC Jr., Walaas SI, Greengard P (1984). DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci 4: 111–124.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press (2005).
Randall PA, Pardo M, Nunes EJ, Lopez Cruz L, Vemuri VK, Makriyannis A et al (2012). Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PloS One 7: e47934.
Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD et al (2009). Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology 150: 707–712.
Schultz W (2002). Getting formal with dopamine and reward. Neuron 36: 241–263.
Segovia KN, Correa M, Lennington JB, Conover JC, Salamone JD (2012). Changes in nucleus accumbens and neostriatal c-Fos and DARPP-32 immunoreactivity during different stages of food-reinforced instrumental training. Eur J Neurosci 35: 1354–1367.
Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M et al (2013). Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol Clin Exp Res 37: 1351–1360.
Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M et al (2011). Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol Psychiatry 69: 642–649.
Svenningsson P, Nairn AC, Greengard P (2005). DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J 7: E353–E360.
Tanda G, Pontieri FE, Di Chiara G (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276: 2048–2050.
Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007). Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64: 1575–1579.
Wahli W, Michalik L (2012). PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab 23: 351–363.
Wise RA (1996). Neurobiology of addiction. Curr Opin Neurobiol 6: 243–251.
Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA et al (2006). Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology 31: 555–562.
Acknowledgements
This study was supported by the University of Camerino (to RC). Dr Demopulos is the Chairman and CEO of Omeros Corporation and Dr Gaitanaris is Chief Scientific Officer of Omeros Corporation. Omeros exclusively controls the intellectual property rights directed to the use of PPARγ receptor agonists for the treatment of addiction and addictive behaviors obtained from the University of Camerino and Dr Ciccocioppo. Dr Ciccocioppo is the inventor on a number of patent applications, which have been assigned to Omeros, relating to the therapeutic use of PPARγ agonists in addiction. He is entitled to receive payments and royalties from Omeros under such licensing arrangement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
de Guglielmo, G., Melis, M., De Luca, M. et al. PPARγ Activation Attenuates Opioid Consumption and Modulates Mesolimbic Dopamine Transmission. Neuropsychopharmacol 40, 927–937 (2015). https://doi.org/10.1038/npp.2014.268
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.268
This article is cited by
-
PPARα and PPARγ are expressed in midbrain dopamine neurons and modulate dopamine- and cannabinoid-mediated behavior in mice
Molecular Psychiatry (2023)
-
CRHCeA→VTA inputs inhibit the positive ensembles to induce negative effect of opiate withdrawal
Molecular Psychiatry (2021)
-
Activation of peroxisome proliferator-activated receptor γ reduces alcohol drinking and seeking by modulating multiple mesocorticolimbic regions in rats
Neuropsychopharmacology (2021)
-
Beta-caryophyllene inhibits cocaine addiction-related behavior by activation of PPARα and PPARγ: repurposing a FDA-approved food additive for cocaine use disorder
Neuropsychopharmacology (2021)
-
Functional observation after morphine withdrawal: effects of SJP-005
Psychopharmacology (2021)