Abstract
Exposure to drugs of abuse lead to both rewarding effects and the subsequent development of negative affects. The progressive dysregulation of both processes is thought to critically contribute to the addictive state. Whereas cocaine-induced maladaptations in reward circuitry have been extensively examined, the cellular substrates underlying negative affect remain poorly understood. This study focuses on the central nucleus of the amygdala (CeA), a brain region that has been implicated in negative affective states upon withdrawal from chronic cocaine use. We observed that the two major types of CeA neurons, low-threshold bursting (LTB) neurons and regular spiking (RS) neurons, exhibited different sensitivity to corticotrophin-releasing factor (CRF), a stress hormone that has been implicated in negative affect during drug withdrawal. Furthermore, LTB and RS neurons developed opposite membrane adaptations following short-term (5 day) cocaine self-administration; the membrane excitability was increased in LTB neurons but decreased in RS neurons. These short-term exposure-induced effects were transient as they were present on withdrawal day 1 but disappeared on withdrawal day 21. However, extended exposure (21 day) led to sustained increase in the membrane excitability of LTB neurons such that it lasted over 21 days into the withdrawal period. These results suggest that CeA neurons can be a cellular target for cocaine to reshape the circuitry mediating negative affects during withdrawal, and that the long-lasting cellular alterations in selective subpopulations of CeA neurons may lead to unbalanced CeA processing, thus contributing to the progressive aggravation of negative affective states during withdrawal from chronic cocaine exposure.
Similar content being viewed by others
INTRODUCTION
Following exposure to drugs of abuse, chronic drug users often experience initial drug-induced euphoric effects, followed by withdrawal-associated negative affective states, such as dysphoria, stress, anxiety, and depression (Anthony et al, 1989; Williamson et al, 1997). Withdrawal-associated negative affect is often modest and likely reversible after short-term drug exposure, but becomes strong and likely irreversible after extended exposure (Koob, 2009). Both animal work and theoretical analyses suggest that withdrawal-associated negative affective states may serve as a mechanism independent of, but complementary to, the positive reinforcing effects of drugs of abuse to promote drug seeking (Koob, 2008; Koob and Le Moal, 1997; Solomon and Corbit, 1974). Despite the apparent theoretical and clinical significance, the molecular and cellular mechanisms underlying withdrawal-associated negative affective states remain poorly understood. The present study examined cocaine-induced alterations in the central nucleus of the amygdala (CeA), a brain region that has been critically implicated in the regulation of negative affective states typically experienced during withdrawal or prolonged drug use (Funk et al, 2006; Heinrichs et al, 1995; Shibata et al, 1982; Wenzel et al, 2011; Xue et al, 2012).
As an important output nucleus of the amygdala, the CeA receives convergent projections from other amygdaloid subregions and projects to the brain stem and hypothalamus for the generation of emotional and motivational responses (Pitkanen et al, 1997). In theory, whether and how these synaptic inputs to the CeA are translated into informational outflow is determined, to a significant degree, by the intrinsic membrane excitability of CeA neurons. The membrane excitability sets the threshold for action potential (AP) initiation and determines the firing frequency once the membrane potential is driven to the threshold by synaptic input. Thus, by regulating the membrane excitability of CeA neurons, exposure to cocaine or other stimuli may reshape the functional output of the CeA to regulate the affective state. Indeed, long-term regulation of membrane excitability is a common and critical mechanism by which exposure to cocaine induces long-term alterations within the brain reward circuitry that mediates the reinforcing effects of drugs of abuse (Huang et al, 2011; Wolf, 2010). However, it remains to be determined whether the membrane excitability of CeA neurons is a cellular target of cocaine exposure.
Here, we focused on two major types of CeA neurons, regular spiking (RS) and low-threshold bursting (LTB) neurons (Chieng et al, 2006; Dumont et al, 2002). We observed that LTB and RS neurons responded differently to acute application of stress-response hormone corticotropin-releasing factor (CRF), suggesting that they may be differentially associated with the development of negative affective states during or after drug exposure. Indeed, following short-term (5 days) cocaine self-administration (SA), the membrane excitability was transiently decreased in RS neurons but increased in LTB neurons. In contrast, extended cocaine SA (21 days) induced persistent (> 21 days) increase in the membrane excitability selectively in LTB neurons. Thus, extended, but not limited, exposure to cocaine triggers long-lasting membrane adaptations in a selective population of CeA neurons. These results may provide a cellular basis for the long-lasting negative affective state during withdrawal from chronic cocaine use.
MATERIALS AND METHODS
Animals and Cocaine SA
Male Sprague Dawley rats were used for all experiments. The rats were purchased at 23–28-day old (Simonson or Harlan Laboratories), and were placed in the home cages for at least a week before experimentation. SA surgery was derived from Mu et al, 2010. Briefly, a silastic catheter (∼5 cm; ID 0.020 inch, OD 0.037 inch) was inserted into the right auricle through the external jugular vein, with the distal end led subcutaneously to the back between the scapulas, and connected to a Quick Connect Harness (SAI Infusion). Rats were allowed to recover for 1–2 weeks. During recovery, the catheter was flushed daily with heparin (10 U/ml, 0.1 ml) and gentamicin (5 mg/ml) in sterile saline to protect against infection and catheter occlusion.
Animals started cocaine SA training on postnatal day (PND) 35–38, 5–8 days after surgery, in operant-conditioning chambers (Med Associates). Each chamber contains an active and an inactive nose poke hole, a cocaine-infusion line controlled by a programmable syringe pump, a conditioned stimulus (CS) light in each nose poke hole, and a house light. On day 1, animals were placed in the chamber for an overnight training session on a fixed ratio (FR) 1 schedule. A nose poke in the active hole resulted in a cocaine infusion (0.75 mg/kg in 0.10 ml) and illumination of a CS light. The CS light remained on for 6 s, whereas a house light was illuminated for 20 s during which active nose pokes were counted but resulted in no further cocaine infusions. At the end of the 20 s, the house light was extinguished, and the next nose poke in the active hole resulted in another cocaine infusion. Nose pokes in the inactive hole had no reinforcement effects but were recorded. Occasionally animals were given a 2nd overnight training if they received <50 cocaine infusions during the 1st overnight training session. Thereafter, rats were allowed to self-administer cocaine for 6 h/day for 5 or 21 consecutive days on a FR1 schedule, followed by withdrawal in home cages.
CeA Slice Preparation
The rat was anesthetized with isoflurane and subsequently perfused transcardially with 4 °C cutting solution (in mM: 135 N-methyl-D-glucamine, 1 KCl, 1.2 KH2PO4, 0.5 CaCl2, 1.5 MgCl2, 20 choline-HCO3, 11 glucose, pH adjusted to 7.4 with HCl, and saturated with 95% O2/5% CO2). The rat was then decapitated; the brain was removed and sliced using a Leica VT1200s vibratome in 4 °C cutting solution. Coronal slices of 250–300 μm thickness were cut such that the preparation contained the CeA. Slices were then incubated in artificial CSF (aCSF) (in mM: 119 NaCl, 2.5 KCl, 1 NaH2PO4, 1.3 MgCl2, 2.5 CaCl2, 26.2 NaHCO3, and 11 glucose, 290 mOsm, saturated with 95% O2/5% CO2) at 37 °C for 30 min and then allowed to recover for 1–2 h at room temperature before electrophysiology. One slice was then transferred from the holding chamber to a submerged recording chamber, where it was continuously superfused with oxygenated aCSF maintained at 31–34 °C.
Electrophysiology
Whole-cell current-clamp recordings were preferentially made from neurons located in the medial CeA (Figure 1a–c) using a MultiClamp 700B amplifier (Molecular Device), with a K+-based internal solution (in mM: 108 K-methanesulfonate, 20 KCl, 10 HEPES, 0.4 EGTA, 2.0 MgCl2, 2.5 MgATP, 0.25 Na3GTP, 7.5 Na2-phosphocreatine, 1 L-glutathione, pH 7.25–7.30; 290 mOsm). At least 5 min after achieving whole-cell configuration, a current-step protocol (from −200 to +400 pA, with a 50 pA increment) was run and repeated. The after-hyperpolarization potential (AHP) was sampled following the first single AP spike. For the CRF experiment, tetrodotoxin (TTX, 1 μM) was bath-applied to block voltage-gated Na+ channels after cell-type identification (LTB vs RS) and remained present as CRF (30 nM) was applied (Giesbrecht et al, 2010). To inhibit CRF receptors, NBI 27914 (500 nM) or astressin 2B (100 nM) was bath-applied for at least 10 min before CRF application (Hahn et al, 2009). TTX, CRF, NBI 27914, and astressin 2B were purchased from R&D Systems, and all other reagents were obtained from Sigma-Aldrich. Cocaine was provided by the Drug Supply Program at NIH NIDA.
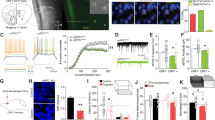
Low-threshold bursting (LTB) and regular spiking (RS) neurons in the central nucleus of the amygdala (CeA). (a, b) Diagram and example image showing the CeA in a coronal slice (Bregma −2.16 mm). Calibration bar, 500 μm. (c) Example image showing the CeA neurons under × 40 objective. Calibration bar, 20 μm. (d) Action potential (AP) in an example LTB neuron with a prominent depolarizing after-potential (DAP; arrow head); minimal DAP in typical RS neurons. (e, f) Membrane properties of example LTB and RS neurons. Under current-clamp mode, step-wise hyperpolarizing and depolarizing currents were injected into the recorded neuron. The example LTB neuron (e) generated spike doublets or triplets at the beginning of the AP train. The burst of spikes was absent in the example RS neuron (f).
Data Acquisition and Analysis
Numbers of cells (n) and animals (m) are presented as ‘n/m’. Unless specified, ‘n’ as the default sample size was used in all statistics. All results are shown as mean±SEM. One or two-factor repeated-measures ANOVA were used in most analyses. For two-factor ANOVA, factor-A was assigned for the treatments (eg, cocaine vs saline) and Factor-B was assigned for current injections. The statistical results were primarily presented in the F- and P-values of the main effect of Factor-A, which was the primary research interest. Degrees of freedom of between (b) and within (w) treatments were presented as F(b,w). Cohen’s d was calculated in some cases to determine the effect size: a value of 0.20 is interpreted as a small effect, 0.50 medium effect, and 0.80 large effect.
RESULTS
The CeA is a complex subregion of the amygdala, largely comprising the medial, lateral, and capsular divisions (Cassell et al, 1986; McDonald, 1982). These divisions could not be readily discerned in unstained slices, but efforts were made to record neurons in the medial CeA (Figure 1a–c). Consistent with previous studies (Chieng et al, 2006; Dumont et al, 2002), most (304 out of 329) neurons we confronted were LTB or RS neurons. Although LTB and RS neurons exhibited similar passive membrane properties, they could be distinguished by their distinct spiking patterns. Upon depolarizing current injections, LTB neurons typically initiated firing by generating spike doublets or triplets (Figure 1e), whereas this bursting pattern was absent in RS neurons (Figure 1f). At near threshold, LTB neurons sometimes fired a single AP followed by a prominent depolarizing after-potential (DAP), whereas the DAP was minimal in RS neurons (Figure 1d; Table 1).
The stress hormone CRF has a critical role in CeA-mediated negative affect associated with drug withdrawal (Koob and Le Moal, 2008). Two prominent ways by which CRF regulates neuronal activity in other brain regions are: (i) to depolarize the membrane potential by inducing a sustained steady-state current, and/or (ii) to enhance the hyperpolarization-activated depolarizing current (Ih current) (Giesbrecht et al, 2010; Qiu et al, 2005; Wanat et al, 2008). Focusing on these two electrophysiological parameters, we next attempted to determine whether LTB and RS neurons responded to CRF differentially. As shown in Figure 2a and b, LTB and RS neurons both responded to acute application of CRF (30 nM) by a sustained depolarizing current (across voltage range: LTB, F(1,310)=23.74, P<0.001; RS, F(1,380)=7.79, P<0.01). However, the size of the sustained current was significantly larger in LTB neurons than in RS neurons (F(1,345)=5.62; P<0.05, Figure 2a and b). Furthermore, pre-application of CRF1 receptor selective antagonist NBI 27914 (500 nM) but not CRF2 receptor antagonist (astressin 2B, 100 nM) abolished the CRF-induced sustained current in LTB neurons (compared with antagonist-only controls; NBI 27914+CRF: F(1,60)=0.11, P=0.75; astressin 2B+CRF: F(1,80)=58.61, P<0.001, Figure 2a). By contrast, in RS neurons, the CRF-induced sustained current was abolished by pre-application of CRF2 receptor selective antagonist astressin 2B, but not NBI 27914 (compared with antagonist-only controls; astressin 2B+CRF: F(1,70)=0.01, P=0.92; NBI 27914+CRF: F(1,70)=5.87, P<0.05; Figure 2b).
Low-threshold bursting (LTB) and regular spiking (RS) neurons in the central nucleus of the amygdala (CeA) respond differently to corticotrophin-releasing factor (CRF). (a) Examples (a1–3) and summaries (a4) showing that in LTB neurons the steady-state currents from the ramp (−100 mV to −55 mV) recording was enhanced by application of CRF (30 nM); this effect was prevented by pre-application of CRF receptor 1-selective antagonist NBI 27914 (NBI; 500 nM), but not CRF receptor 2-seletive antagonist astressin 2B (astressin; 100 nM). (b) Examples (b1–3) and summaries (b4) showing that the steady-state currents in RS neurons were enhanced by application of CRF; this effect was prevented by pre-application of astressin but not NBI. (c) Examples (c1–3) and summaries (c4, 5) showing that Ih currents in LTB neurons were enhanced by application of CRF, and this effect was prevented by pre-application of NBI, but not astressin. Inward currents (c1–3 middle) were evoked by voltage steps (−65 to −115 mV with an increment of 10 mV; c1–3 upper). The amplitudes of Ih currents (c1–3 bottom) were assessed as the amplitude differences between the steady-state currents (indicated by arrows) and the initial phases of the currents (horizontal bar). The effects of CRF and antagonists were assessed using the Ih current evoked at −115 mV (c5). (d) Examples and summaries showing that Ih currents were not affected by application of CRF. Tetrodotoxin (TTX; 1 μM) was present throughout CRF application. *P<0.05; **P<0.01.
We next examined the Ih current. Both LTB and RS CeA neurons exhibited the Ih current at basal conditions, although the averaged amplitude appeared to be higher in RS neurons (Figure 2c and d). Application of CRF significantly enhanced the Ih current selectively in LTB but not RS neurons (LTB, F(1,300)=17.99, P<0.001; RS, F(1,380)=0.20; P=0.66; Figure 2c and d). Moreover, the CRF-induced increase in Ih current in LTB neurons was abolished by pre-application of CRF1-selective antagonist NBI 27914, but not CRF receptor 2-selective antagonist astressin 2B (compared with antagonist-only controls; NBI 27914+CRF: F(1,70)=0.80, P=0.40; astressin 2B+CRF: F(1,70)=17.82, P<0.01; Figure 2c5).
These results suggest that LTB and RS neurons respond differently to CRF, likely through different CRF receptors. Given that sustained steady-state currents typically involve slowly-inactivating voltage-gated ion channels including HCN channels (Biel et al, 2009; Giesbrecht et al, 2010; Qiu et al, 2005), these results also suggest differential coupling mechanisms of CRF signaling to ion channels in LTB and RS neurons. Thus, LTB and RS neurons may undergo different membrane adaptations following drug exposure and withdrawal. We therefore examined and compared these two types of CeA neurons for their cocaine-induced cellular adaptations.
To examine the effect of short-term exposure to cocaine, we trained the rats with a 5-day cocaine SA procedure (0.75 mg/kg/infusion × 6 h/day × 5 days), through which animals acquired active cocaine taking (Figure 3a). On withdrawal day 1 (animals were 41–44 days old), we measured the membrane excitability of LTB and RS neurons by recording evoked APs, as established previously (Dong et al, 2006; Ishikawa et al, 2009; Mu et al, 2010). LTB and RS neurons exhibited significant but opposing adaptations. Compared with saline controls, the membrane excitability was increased in LTB neurons (F(1,282)=6.61; P<0.05, Cohen’s d=0.34; animal-based statistics, F(1,72)=5.28, P<0.05) (Figure 3b and d), but decreased in RS neurons (F(1,324)=10.83; P<0.01, Cohen’s d=0.46; animal-based statistics, F(1,72)=8.95, P<0.05) (Figure 3f and h). Accompanying the increased membrane excitability of LTB neurons, there was a decrease of the rheobase current, defined as the minimal current necessary to produce an AP (t(47)=2.71, P<0.01; Table 1). On the other hand, upon hyperpolarizing current injections, LTB but not RS neurons from cocaine-exposed rats exhibited significantly larger membrane potential drops than that of the control group (LTB, F(1,180)=5.52, P<0.05; RS, F(1,212)=0.85, P=0.36; Figure 3c), suggesting greater membrane resistance. In addition, in RS neurons the hyperpolarization-induced depolarization (sag) was significantly decreased in the cocaine group (t(52)=2.74, P<0.01; Figure 3g; Table 1), a change not observed in LTB neurons (t(45)=0.35, P=0.73). This reduction in the ‘sag’ may contribute to cocaine-induced decrease in AP firing in RS neurons by reducing the depolarizing drive toward the AP threshold following membrane repolarization. No significant changes in the AP threshold (Table 1) or the % of RS/LTB neurons were detected (Figure 3e). These results suggest that exposure to cocaine may differentially affect the membrane properties of LTB and RS neurons at both depolarizing and hyperpolarizing potentials.
Short-term exposure to cocaine differentially alters the membrane excitability of low-threshold bursting (LTB) and regular spiking (RS) neurons. (a) Procedure diagram showing 5-day cocaine self-administration (SA) and 1-day withdrawal (w/d), and acquisition of cocaine SA. (b) Example action potential (AP) trains from LTB neurons of a saline- or cocaine-treated animal upon current injections. (c) Summary of the steady-state membrane potentials upon hyperpolarizing current injections showing significantly increased membrane resistance in LTB but not RS neurons. Inset shows the measurement of steady-state potential. (d) Summary showing increased membrane excitability of LTB neurons after short-term withdrawal from cocaine SA. (e) % Of all recorded central nucleus of the amygdala (CeA) neurons that exhibited LTB, RS, or other signature AP firing patterns. (f) Example AP trains from RS neurons of a saline- or cocaine-treated animal upon depolarizing or hyperpolarizing current injections. (g) Summary of hyperpolarization-induced depolarization (sag) in LTB and RS neurons by −200 pA current injection showing significant decrease in the sag potential in RS but not LTB neurons. Inset shows the sag potential measurement. (h) Summary showing decreased membrane excitability of RS neurons after short-term withdrawal from cocaine SA. *P<0.05; **P<0.01.
To examine whether these cocaine-induced membrane adaptations were long-lasting, separate groups of rats received the same cocaine procedure but with 21-day withdrawal (Figure 4a). At this withdrawal time point (animals were 61–64 days old), a trend toward increase in the membrane excitability was still present in LTB neurons (F(1,348)=3.47; P=0.07; Figure 4b and d), whereas the decrease in the membrane excitability of RS neurons disappeared entirely (F(1,294)=0.06; P=0.81; Figure 4f and h). The differences in membrane resistance (LTB, t(58)=1.08, P=0.29; RS, t(46)=0.25, P=0.80) and sag potentials (LTB, t(55)=0.06; P=0.95, RS, t(49)=0.24; P=0.81; Table 1) also became not significant (Figure 4c and g). Furthermore, no significant changes in the rheobase current, AP threshold, or the % of RS/LTB neurons were detected (Table 1; Figure 4e). Thus, following short-term exposure to cocaine, both LTB and RS neurons underwent membrane adaptations, but these changes were restored or reversed over long-term withdrawal.
The effect of short-term cocaine exposure on the membrane excitability of central nucleus of the amygdala (CeA) neurons is transient. (a) Procedure diagram showing 5-day cocaine self-administration (SA) and 21-day withdrawal (w/d), and acquisition of cocaine SA during the training period. (b) Example action potential (AP) trains from low-threshold bursting (LTB) neurons of a saline- or cocaine-treated animal upon current injections. (c) Summary of steady-state membrane potentials upon hyperpolarizing current injections. Inset shows the measurement of steady-state potential. (d) Summary showing a trend (but non-significant) toward an increase in the membrane excitability of LTB neurons after 21-day withdrawal from cocaine SA. (e) % Of all recorded CeA neurons that exhibited LTB, regular spiking (RS) or other signature AP firing patterns. (f) Example AP trains from RS neurons of a saline- or cocaine-treated animal upon current injections. (g) Summary of hyperpolarization-induced depolarization (sag) in LTB and RS neurons. Inset shows the sag potential measurement. (h) Summary showing no detectable changes in the membrane excitability of RS neurons after 21-day withdrawal from cocaine SA.
Short-term and long-term cocaine experiences produce very different behavioral and cellular consequences. In animal models, several addiction-characteristic behaviors, such as compulsive cocaine seeking (Deroche-Gamonet et al, 2004; Vanderschuren and Everitt, 2004) and escalation of cocaine taking (Ahmed and Koob, 1998), are only induced by extended cocaine use. Moreover, cocaine-induced negative or opponent affective states are often transient following short-term or low doses of exposure but become persistent after extended exposure (Kenny et al, 2003; Markou and Koob, 1991). We thus asked whether cocaine-induced membrane adaptations in CeA neurons may stabilize upon extended exposure to cocaine. To test this, we employed a 21-day cocaine SA procedure, through which animals exhibited the characteristic escalating cocaine taking (Figure 5a). After 21 days of withdrawal, LTB neurons exhibited lower threshold for AP initiation (t(29)=2.10; P<0.05; Figure 5c; Table 1) and enhanced membrane excitability in cocaine-exposed animals than in saline controls (F(1,174)=4.24; P<0.05; Figure 5b and d). No significant changes were detected in the membrane resistance of LTB neurons (t(29)=0.21; P=0.83), suggesting that long-term and short-term cocaine exposure enhances membrane excitability via different mechanisms. In RS neurons, no significant changes were detected in the membrane excitability (F(1,144)=0.69, P=0.41), although a trend toward decrease was observed (Figure 5h). This trend, even if it holds as a real effect, is rather small (Cohen’s d=0.17), and thus was not pursued further. RS neurons also exhibited significantly enhanced AHP following AP firing in the cocaine group (t(24)=2.09, P<0.05; Figure 5g), which has been shown in various circumstances to dampen the membrane excitability (Mu et al, 2010; Oh et al, 2003; Saar et al, 1998). Collectively, the changes in the membrane excitability of CeA neurons were apparently more long-lasting after extended exposure to cocaine.
Extended exposure to cocaine persistently alters the membrane excitability of central nucleus of the amygdala (CeA) neurons. (a) Procedure diagram showing 21-day cocaine self-administration (SA) training and 21-day withdrawal (w/d), and acquisition of cocaine SA during the training period. (b) Example action potential (AP) trains from low-threshold bursting (LTB) neurons of a saline- or cocaine-treated animal upon depolarizing current injections. (c) LTB but not regular spiking (RS) neurons exhibited significant decrease in AP threshold. (d) Summary showing a significant increase in the membrane excitability of LTB neurons after 21-day withdrawal from extended exposure to cocaine. (e) % Of all recorded CeA neurons that exhibited LTB, RS, or other signature AP firing patterns. (f) Example AP trains from RS neurons of a saline- or cocaine-treated animal upon depolarizing current injections. (g) Summary showing a significant increase in after-hyperpolarization (AHP) in RS but not LTB neurons. (h) Summary showing a trend (but not significant) toward a decrease in the membrane excitability of RS neurons after 21-day withdrawal from extended exposure to cocaine. *P<0.05.
DISCUSSION
Aiming to explore the cellular mechanisms underlying long-lasting negative affective state during cocaine withdrawal, the present study focuses on LTB and RS neurons, the two major neuronal types in the CeA. Our results show that LTB and RS neurons exhibited transient adaptations in membrane excitability following short-term exposure to cocaine, and some of these adaptations became persistent following extended exposure. These results suggest that the membrane properties of CeA neurons can be targeted by drugs of abuse to produce long-lasting cellular and behavioral alterations.
Differential Effects of Cocaine on Different CeA Neurons
Despite considerable cytochemical and morphological similarities (Chieng et al, 2006), LTB and RS CeA neurons are distinctly different from each other for their membrane properties and cellular responses to CRF signaling. They also exhibited opposite cocaine-induced membrane adaptations. Anatomically, the CeA receives inputs from the thalamus and lateral hypothalamus, in addition to intra-amygdala projections, and projects mainly to the hypothalamus and brain stem (Jolkkonen and Pitkanen, 1998; Tokita et al, 2010). Although it is tempting to speculate that these two types of neurons relay different afferent/efferent projections, supporting evidence is lacking. Nonetheless, the different effects of cocaine on these two major types of CeA neurons suggest different roles for these neurons in cocaine-induced emotional and motivational responses. In addition to the well-established role of CeA in the opponent-process of cocaine action, increasing evidence suggests that the CeA also contributes to the positive reinforcing effect of drugs of abuse, such that infusion of amphetamine directly into the CeA produces a conditioned place preference (O'Dell et al, 1999). A parsimonious interpretation of these dual roles of CeA is that two different types of neurons in the CeA are differentially involved.
Short-Term vs Extended Exposure
It is worth noting that the short-term and long-term cocaine exposure experiments cover the age range of ∼PND 35 (start training) through ∼PND 56 (finish), during which there may be a transition from adolescence to young adulthood with accompanying differences in the vulnerability to psychostimulants. Nevertheless, extensive evidence suggests that the duration of exposure is a critical factor for drugs of abuse to induce the addictive state. This is exemplified in a seminal study in which experimental animals exhibited escalating cocaine intake only after a prolonged, but not short, period of extended access to cocaine, and this escalation persists throughout long-term withdrawal (Ahmed and Koob, 1998). Escalation of cocaine intake, which is to some extent similar to the binge intake of cocaine in human addicts, can be interpreted at the behavioral and theoretical levels as a result of an integration of progressive decreases in primary rewarding effect of drugs and increases in negative affect (Koob and Le Moal, 2008). Under this scenario, brain regions mediating reward as well as those mediating negative affect processing may have undergone adaptive changes following prolonged cocaine use. Whereas several important prolonged exposure-specific adaptations have been identified in the reward pathway (Wolf, 2010), identification of the counterparts in brain regions associated with negative affects has just started. One candidate mechanism is the CeA CRF system, which is hypothesized to mediate negative affects during drug withdrawal (Koob, 2009, 2010; Koob and Zorrilla, 2010). Specifically, CRF is increased in CeA following extended but not short-term access to cocaine (Zorrilla et al, 2012), and blocking CeA CRF signaling by CRF receptor antagonists blocks excessive drug intake (Funk et al, 2006; Koob, 2009). When applied acutely, CRF enhances the membrane excitability of neurons both in the hippocampus and in the basal lateral amygdala (Aldenhoff et al, 1983; Blank et al, 2003; Giesbrecht et al, 2010), and similar effect was observed in CeA neurons (Figure 2). Notably, CeA LTB neurons, which showed significantly larger CRF responsiveness than RS neurons (Figure 2), also showed more persistent changes in the membrane excitability following extended cocaine exposure (Figure 5).
Without distinguishing neuronal types, a previous study shows that during cocaine withdrawal CRF-induced potentiation of excitatory synaptic transmission to CeA neurons is enhanced (Pollandt et al, 2006), an effect that may result in upregulation of glutamate receptors (Lu et al, 2005; Pollandt et al, 2006). This effect, if concurrent with enhanced membrane excitability on CeA LTB neurons, would predict increased CeA activity during cocaine withdrawal. This prediction is partially supported by recent imaging studies showing increased amygdala activity upon cocaine-associated cues in chronic cocaine users but not in naive controls (Childress et al, 1999; Grant et al, 1996). The resolution of these studies, however, cannot readily separate CeA from other amygdala subregions, leaving this intriguing possibility unchecked.
In summary, long-lasting changes in the membrane properties of CeA neurons following prolonged exposure to cocaine may represent a distinct set of drug-induced adaptations that mediate the development of negative affective states in drug withdrawal.
FUNDING AND DISCLOSURE
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers DA030379, DA023206, DA029565, and DA031551. The authors declare no conflict of interest.
References
Ahmed SH, Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300.
Aldenhoff JB, Gruol DL, Rivier J, Vale W, Siggins GR (1983). Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science 221: 875–877.
Anthony JC, Tien AY, Petronis KR (1989). Epidemiologic evidence on cocaine use and panic attacks. Am J Epidemiol 129: 543–549.
Biel M, Wahl-Schott C, Michalakis S, Zong X (2009). Hyperpolarization-activated cation channels: from genes to function. Physiol Rev 89: 847–885.
Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J (2003). Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci 23: 700–707.
Cassell MD, Gray TS, Kiss JZ (1986). Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol 246: 478–499.
Chieng BC, Christie MJ, Osborne PB (2006). Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol 497: 910–927.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Deroche-Gamonet V, Belin D, Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science 305: 1014–1017.
Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ et al (2006). CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci 9: 475–477.
Dumont EC, Martina M, Samson RD, Drolet G, Pare D (2002). Physiological properties of central amygdala neurons: species differences. Eur J Neurosci 15: 545–552.
Funk CK, O'Dell LE, Crawford EF, Koob GF (2006). Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26: 11324–11332.
Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, Colmers WF (2010). Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J Neurosci 30: 16970–16982.
Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C et al (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93: 12040–12045.
Hahn J, Hopf FW, Bonci A (2009). Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci 29: 6535–6544.
Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L (1995). Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol 6: 74–80.
Huang YH, Schluter OM, Dong Y (2011). Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behav Brain Res 216: 9–18.
Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM et al (2009). Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci 29: 5820–5831.
Jolkkonen E, Pitkanen A (1998). Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol 395: 53–72.
Kenny PJ, Polis I, Koob GF, Markou A (2003). Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur J Neurosci 17: 191–195.
Koob GF (2008). A role for brain stress systems in addiction. Neuron 59: 11–34.
Koob GF (2009). Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 (Suppl 1): 18–31.
Koob GF (2010). The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314: 3–14.
Koob GF, Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science 278: 52–58.
Koob GF, Le Moal M (2008). Addiction and the brain antireward system. Annu Rev Psychol 59: 29–53.
Koob GF, Zorrilla EP (2010). Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Current Opin Invest Drugs 11: 63–71.
Lu L, Dempsey J, Shaham Y, Hope BT (2005). Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J Neurochem 94: 161–168.
Markou A, Koob GF (1991). Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4: 17–26.
McDonald AJ (1982). Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 208: 401–418.
Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA et al (2010). Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci 30: 3689–3699.
O'Dell LE, Sussman AN, Meyer KL, Neisewander JL (1999). Behavioral effects of psychomotor stimulant infusions into amygdaloid nuclei. Neuropsychopharmacology 20: 591–602.
Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF (2003). Watermaze learning enhances excitability of CA1 pyramidal neurons. J Neurophysiol 90: 2171–2179.
Pitkanen A, Savander V, LeDoux JE (1997). Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523.
Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP et al (2006). Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci 24: 1733–1743.
Qiu DL, Chu CP, Shirasaka T, Tsukino H, Nakao H, Kato K et al (2005). Corticotrophin-releasing factor augments the I(H) in rat hypothalamic paraventricular nucleus parvocellular neurons in vitro. J Neurophysiol 94: 226–234.
Saar D, Grossman Y, Barkai E (1998). Reduced after-hyperpolarization in rat piriform cortex pyramidal neurons is associated with increased learning capability during operant conditioning. Eur J Neurosci 10: 1518–1523.
Shibata K, Kataoka Y, Gomita Y, Ueki S (1982). Localization of the site of the anticonflict action of benzodiazepines in the amygdaloid nucleus of rats. Brain Res 234: 442–446.
Solomon RL, Corbit JD (1974). An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev 81: 119–145.
Tokita K, Inoue T, Boughter JD Jr (2010). Subnuclear organization of parabrachial efferents to the thalamus, amygdala and lateral hypothalamus in C57BL/6J mice: a quantitative retrograde double labeling study. Neuroscience 171: 351–365.
Vanderschuren LJ, Everitt BJ (2004). Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305: 1017–1019.
Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A (2008). Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol 586: 2157–2170.
Wenzel JM, Waldroup SA, Haber ZM, Su ZI, Ben-Shahar O, Ettenberg A (2011). Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology 217: 221–230.
Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J (1997). Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend 44: 87–94.
Wolf ME (2010). The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33: 391–398.
Xue Y, Steketee JD, Sun W (2012). Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. Eur J Neurosci 35: 775–783.
Zorrilla EP, Wee S, Zhao Y, Specio S, Boutrel B, Koob GF et al (2012). Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addict Biol 17: 300–308.
Author information
Authors and Affiliations
Corresponding authors
Additional information
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Rights and permissions
About this article
Cite this article
Chen, B., Ma, YY., Wang, Y. et al. Cocaine-Induced Membrane Adaptation in the Central Nucleus of Amygdala. Neuropsychopharmacol 38, 2240–2248 (2013). https://doi.org/10.1038/npp.2013.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.124
Keywords
This article is cited by
-
Synaptic mechanisms underlying persistent cocaine craving
Nature Reviews Neuroscience (2016)