Abstract
Benztropine (BZT) analogs, a family of high-affinity dopamine transporter ligands, are molecules that exhibit pharmacological and behavioral characteristics predictive of significant therapeutic potential in cocaine addiction. Here, we examined in mice the effects of 3α-[bis(4′-fluorophenyl)metoxy]-tropane (AHN-1055) on motor activity, conditioned place preference (CPP) and c-Fos expression in the striatum. AHN-1055 produced mild attenuation of spontaneous locomotor activity at a low dose (1 mg/kg) and weak stimulation at a higher dose (10 mg/kg). In parallel, the BZT analog significantly increased c-Fos expression in the dorsolateral caudoputamen at the high dose, whereas producing marginal decreases at low and moderate doses (1, 3 mg/kg) in both dorsal and ventral striatum. Interaction assays showed that cocaine's ability to stimulate locomotor activity was decreased by AHN-1055 treatment, but not by treatment with D-amphetamine. Such reduced ability did not result from an increase in stereotyped behavior. Another dopamine uptake inhibitor, nomifensine, decreased cocaine-induced locomotor activity but evoked by itself intense motor stereotypies. Remarkably, the BZT analog dose-dependently blocked cocaine-induced CPP without producing CPP when given alone, and blocked in conditioned mice cocaine-stimulated early-gene activation in the nucleus accumbens and dorsomedial striatum. These observations provide evidence that AHN-1055 does not behave as a classical psychomotor stimulant and that some of its properties, including attenuation of cocaine-induced striatal c-Fos expression, locomotor stimulation, and CPP, support its candidacy, and that of structurally related molecules, as possible pharmacotherapies in cocaine addiction.
Similar content being viewed by others
INTRODUCTION
There is a growing medical need for effective medications for cocaine addiction, a relapsing disorder with major implications for the affected individuals and society. The wide array of psychological complications associated with cocaine abuse and the lack of specific pharmacotherapies for the disease has fuelled the search for novel treatments that might prevent the effects of cocaine in the brain, protect against relapse to drug seeking in abstinent addicts, or both (Dutta et al, 2003; Karila et al, 2008; Sofuoglu and Kosten, 2005; Kosten and Owens, 2005). Although actions at noradrenergic, serotonergic, and cholinergic synapses are likely to make a contribution, accumulated evidence implicates the dopamine transporter (DAT) in the induction of the psychomotor stimulant effects of cocaine. The subjective effects of cocaine vary primarily as a function of the rate of DAT occupancy by cocaine and the speed of cocaine's delivery into the brain (Volkow et al, 2000; Volkow et al, 1996), suggesting that the pharmacokinetic/dynamic characteristics of cocaine and other psychoactive compounds that share DAT activity might be critical for their addictive properties.
Molecular models of DAT binding have shown that dopamine, cocaine, and amphetamine binding sites extensively overlap, making the design of antagonists, which do not themselves block dopamine uptake, highly troublesome (Beuming et al, 2008; Indarte et al, 2008). Nonetheless, other molecular studies suggested that differential modes of interaction with the DAT lead to specific conformational alterations of the transporter (Chen et al, 2004; Chen and Reith, 2007; Loland et al, 2008; Ukairo et al, 2005; Dar et al, 2005), partially accounting for the dissimilar effects of different DAT inhibitors. Moreover, evidence based on structure–activity relationship studies indicated that various classes of DAT ligands differ fundamentally in pharmacokinetic/dynamic properties, such that their functional activities are not predicted by their binding affinities in vitro at the DAT (Vaughan et al, 1999; Katz et al, 2001). Finally, several novel DAT inhibitors seemed to lack strong cocaine-like behavioral effects in animal models of addiction (Desai et al, 2005b; Katz et al, 2004). Therefore, the design of compounds with DAT activity with potential therapeutic applications is theoretically feasible, in view that the relationship among chemical structure, binding profile at the DAT and behavioral activity is not straightforward. This concept supports the rationale for agonist or replacement therapy in cocaine addiction by means of DAT interference (Grabowski et al, 2004; Rothman, 1990; Rothman et al, 2008).
N-substituted benztropine (BZT) analogs are efficacious dopamine uptake inhibitors with pharmacological and functional characteristics that differ substantially from classical stimulants, such as cocaine. These agents have high affinity for the DAT and inhibit dopamine uptake (Agoston et al, 1997b; Katz et al, 2001). Further, BZT analogs display rates of DAT occupancy slower than that of cocaine (Desai et al, 2005a) and produce increases in extracellular dopamine levels over prolonged periods of time, by contrast to the sharp and transient elevations produced by cocaine (Raje et al, 2003; Raje et al, 2005; Tanda et al, 2005). These features complement their weak or limited capacity to induce cocaine-like behaviors, such as locomotor stimulation and conditioned place preference (CPP) (Desai et al, 2005b; Katz et al, 2004; Li et al, 2005), and support the claim that they might offer a lead for the design of efficacious replacement medications for cocaine addiction.
These experiments were aimed at characterizing the functional interactions between cocaine and one such BZT derivative, 3α-[bis(4′-fluorophenyl)metoxy]-tropane (AHN-1055). We selected this compound because it shows high affinity for the DAT as well as equally effective antagonistic actions at muscarinic M1 receptors (Katz et al, 1999; Katz et al, 2004). The latter feature of the BZT analog could contribute to effectively antagonize the actions of cocaine (Carrigan and Dykstra, 2007; Tanda et al, 2007), although this issue remains controversial (Tanda and Katz, 2007). To determine the extent to which AHN-1055 was able to influence the stimulant and rewarding effects of cocaine, and to gain insight into its possible application as a pharmacotherapy for cocaine addiction, we studied the effects of the BZT analog, administered alone and in combination with cocaine, on c-Fos induction in the striatum, locomotor activity, stereotypy, and place conditioning.
MATERIALS AND METHODS
Subjects
Male Swiss OF-1 mice (N=312), aged 5–6 weeks and weighting 22–26 g (Charles River, Barcelona, Spain) served as subjects. Mice were housed in groups of four subjects after arrival at the laboratories and were allowed 4–7 days to acclimatize to the animal facility before experiments began. The housing room was maintained under constant conditions of temperature (21±2°C) and humidity (50±5%) and was kept on a 12 h light–dark cycle (lights on at 0900 hours). Food and water were provided ad libitum. All experiments were carried out in accordance with the current European directives regulating animal experimentation (86/609/ECC) and were approved by the Ethical Committee (Faculty of Pharmacy) of the University of Valencia.
Pharmacological Treatments
AHN-1055 was synthesized as described earlier (Agoston et al, 1997a). Purity of the product was assessed by magnetic resonance, exceeding 98%. AHN-1055 was dissolved in 0.9% saline, sonicated for complete solubilization and injected at doses of 0, 1, 3, and 10 mg/kg i.p. Dose selection for the BZT analog was based on pilot experiments. Doses higher than 10 mg/kg (20 and 30 mg/kg) were lethal in some mice, when combined with cocaine and were not used in these experiments. Cocaine HCl, D-amphetamine sulfate and nomifensine (Sigma-Aldrich, Gillingham, UK) were dissolved in 0.9% saline and injected at doses of 15, 4, and 20 mg/kg i.p., respectively. Doses for cocaine, D-amphetamine and nomifensine were selected based on preliminary dose–response locomotor activity and/or CPP assays performed in our laboratories. All compounds were prepared fresh daily and were injected at a volume of 10 ml/kg.
Behavioral Assays
Locomotor activity assays were performed in Perspex boxes (53 × 28 × 15 cm). Mice were habituated to the boxes for 20 min before treatments were administered. Two different experiments were carried out. In the first experiment, mice (n=6–8 per group) were distributed into four experimental groups receiving increasing doses of AHN-1055 (0, 1, 3, and 10 mg/kg i.p.). The distance travelled, as a measure of locomotor activity, was automatically recorded during 2 h in bins of 5 min. Mice were monitored with a video-track system with image analysis software (Viewpoint 2.5, Champagne au Mont D'Or, France) that provided unbiased information regarding position, velocity, trajectory, and other relevant behavioral parameters. An additional experimental group was treated with cocaine (15 mg/kg i.p.) and locomotor activity was recorded during 1 h in bins of 5 min. This group was used for comparison purposes in the locomotor and early-gene assays, as this dose of cocaine was utilized throughout the experiments. In the second experiment, we studied the interactions of AHN-1055 (10 mg/kg i.p.), D-amphetamine (4 mg/kg i.p.), and nomifensine (20 mg/kg i.p.) with cocaine (15 mg/kg i.p.). One day before the administration of drug challenges, mice were habituated for 20 min to the same Perspex chambers used in the earlier experiment. The animals were assigned to one of six experimental conditions receiving saline, AHN-1055, D-amphetamine, or nomifensine as a pretreatment followed by cocaine or saline. To monitor the possible induction of long-term effects of the treatments (that is, locomotor sensitization and stereotypy), the drugs were administered during 5 consecutive days. The AHN-1055 pretreatment was given 1 h before cocaine because of the lasting effects of AHN-1055 on dopamine overflow in the striatum (Raje et al, 2005) and locomotor activity, as evidenced in these experiments. The pretreatment with D-amphetamine was given 1 h before cocaine so as to match the protocol for AHN-1055. In our pilot studies, nomifensine seemed to induce short-lived locomotor effects compared with AHN-1055 and D-amphetamine. Thus, we administer it 10 min before cocaine challenge. Pretreatments were administered in the animal facility. Cocaine or saline were given immediately before the mice were placed in the activity chambers. Locomotor activity was monitored during 20 min, thus capturing the fast-onset boost of locomotor activity produced by cocaine treatment. On completion of the test, two trained observers blind to the experimental treatments assessed stereotyped behavior using a 10-point rating scale. Independent assessments of motor stereotypy were made on the five drug sessions. Scores were not accumulated for all sessions. Mice were observed simultaneously for 10 min and a score was agreed upon for each subject. The following behaviors were studied: stereotyped running and rearing (a repetitive pattern of route selection that differs from flexible, variable exploratory running), stereotyped sniffing (persistent head-down sniffing behavior), circling (repetitive 360° turns), compulsive checking (a pattern of repetitive short episodes of sniffing while running along the walls of the apparatus), and stereotyped head movements (uncontrollable head-bobbing movements). Distinct behavioral dimensions, including intensity (number of alternative responses emitted, as an indirect measure of flexibility), frequency (number of responses emitted per unit time), duration (time spent performing the most dominant response), and spatial distribution (degree to which behaviors are spatially confined, as a indirect measure of behavioral focus) were taken into account (Canales and Graybiel, 2000). The responses were rated from 1 to 10 for each of the four dimensions. The assessment consisted in an average of the four measures, rounding to the nearest natural number. Ratings varied from 1 to 10 according to the following parameters: 1, absent; 2, very weak-occasional; 3, weak; 4, moderate; 5, moderate-to-intense; 6, intense; 7, very intense; 8, severe; 9, very severe; and 10, extreme.
Conditioned place preference was carried out in chambers made of Perspex consisting of two equally sized compartments (20 × 18 × 25 cm) interconnected by a rectangular corridor. One of the compartments had black walls and white circles and a metal floor with small-perforated holes. The other compartment had black walls with white stripes and a metal floor with a grid-like pattern. The connecting corridor had transparent walls and a Perspex floor. The apparatus was provided with guillotine doors to allow the confinement of the mice in the compartments during drug-conditioning sessions. The place conditioning procedure consisted of three phases: preconditioning, conditioning, and postconditioning. During preconditioning, mice were habituated to the apparatus for 15 min in 2 consecutive days, the last of which (preconditioning session) was taken as baseline. The movements and location of the mice in the CPP apparatus were monitored using video tracking and software (Viewpoint 2.5, Champagne au Mont D'Or, France) that provided measures of both time spent in each compartment and locomotor activity, estimated as distance travelled. Mice that spent more than 70% of the time in one of the compartments during baseline (n=21, 18% of the sample) were excluded from the study. Mice were assigned to eight experimental groups (n=10–14) receiving saline or AHN-1055 (1, 3, and 10 mg/kg) as a pretreatment followed by saline or cocaine (15 mg/kg) 1 h later. Conditioning was performed over 8 consecutive days, alternating drug sessions with control sessions in which mice received saline injections. During conditioning, the treatments were administered and the mice were confined individually in one of the compartments during 30 min. Treatments and compartments (circles and stripes) were counterbalanced. The postconditioning session was performed 24 h after the last-conditioning session. Mice were placed in the CPP apparatus in a drug-free state and were allowed to explore it for 15 min with the guillotine doors removed. The time spent in each of the compartments was recorded. For analysis purposes, the time spent by the mice in either compartment was summated and expressed as a percentage of the total time spent in the two target compartments. The relative change induced by the conditioning treatments in the preference for one compartment or the other (preconditioning vs postconditioning tests) was estimated as the ratio between the percentage of time spent in the drug-paired compartment and the time spent in the vehicle-paired compartment (Hernandez-Rabaza et al, 2008). An additional set of animals (n=63) was exposed to the same pharmacological treatments and underwent conditioning but was killed after the last-conditioning session. The brains of these mice were used for c-Fos immunocytochemistry.
Immunocytochemistry and Microscopy
Mice were transcardially perfused under pentobarbital anesthesia (100 mg/kg, i.p.) with 0.9% saline followed by 4% paraformaldehyde in phosphate buffer. Mice used for AHN-1055 dose–response assay were killed 2 h after the drug challenge and mice undergoing drug conditioning in the CPP asays were killed 30 min after last-conditioning session. Brains were removed, postfixed and cut in coronal sections (35 μm) on a cryostat. We used a single antigen immunocytochemistry against c-Fos (1 : 5000, Calbiochem, La Jolla, CA), as described earlier (Canales and Graybiel, 2000; Rodriguez-Alarcon et al, 2007). Briefly, endogenous peroxidase activity was quenched with 3% H2O2, and sections were treated with 5% normal goat serum and were incubated overnight with the c-Fos antibody. Sections were exposed to secondary antibody (goat anti-rabbit IgG, Vector Laboratories, Burlingame, CA) and to HRP-conjugated streptavidin (1 : 5000, Vector Laboratories, Burlingame, CA). To reveal antigenic sites, sections were exposed to diaminobenzidine–H2O2 complex with nickel (NiSO4) intensification, which produced a nuclear black reaction product. Controls were performed in which the primary antibody was omitted from the protocol. Sections were mounted with Entellan (Merck, Darmstadt, Germany) and coverslipped. High-resolution photomicrographs were taken with an optical microscope (Nikon Eclipse E800) through the nucleus accumbens (N Acc), and the medial (DMst) and lateral (DLst) sectors of the caudoputamen. Only sections ranging from 1.10–1.35 mm anterior to bregma (Paxinos and Franklin, 2001) were considered for all regions under investigation. For the shell region of nucleus accumbens (N Acc shell) the counting frame was 0.06 mm2 and was placed in the shell region using the most medial part of the anterior commissure as a vertical guide for the right side of the frame. For the nucleus accumbens core (N Acc core), DMst and DLst, the counting frame was adjusted to the contour of the anterior commissure, the lateral ventricle and the corpus callosum, respectively. The size of the counting frame for the N Acc core was 0.18–0.20 mm2. For both DMst and DLst, the counting frame was 0.30–0.34 mm2. Digital photomicrographs were taken after equalization using the corpus callosum as a blank and were examined with image analysis software (ImageTool, UTHSCSA). Counting frames were applied to the regions under investigation and threshold intensity was set manually within a constant range to remove background and faint stain. Positively immunolabelled cells were counted blind to the experimental treatments and results were expressed as density of c-Fos-positive cells (cells/mm2) for each of the regions studied.
Statistical Analysis
Parametric data were analyzed by ANOVA followed by Newman–Keuls (N–K) post hoc comparisons using the overall sampling error from the ANOVA as denominator. For the analysis of non-parametric observations, we followed the procedure of Conover and Iman (1981) involving rank transformations and ANOVA (Canales et al, 2000). Games–Howell (G–H) post hoc comparisons were made after ANOVA, thus preserving the principles of normal distribution and homogeneity of variances. Statistical significance was set at α=0.05 per experiment.
RESULTS
AHN-1055 is Unlike Cocaine in Early-Gene and Locomotor Activity Assays
To test and characterize the effects of AHN-1055 on locomotor activity and to correlate such changes with variations in striatal neuronal activation, mice received different doses of the BZT analog before being placed in the activity chambers. The results of these experiments are shown in Figure 1. To analyze the locomotor activity data, ANOVA was carried out with one between-subjects factor, treatment, with four levels (doses of AHN-1055; 0, 1, 3, and 10 mg/kg) and one within-subjects factor, time course, with 24 levels (5 min bins over 120 min of test). The results indicated a significant effect of the treatment factor (F=3.927, p=0.024), which was attributable to differences between the high and the low dose of AHN-1055, as revealed by post hoc comparisons. When the accumulated values for the session were examined, neither the values for the high dose of the BZT analog nor those for the low dose differed significantly from baseline (Figure 1b), although mean differences approached critical differences in N–K tests. When we explored the effects of the treatments across time, ANOVA indicated a significant interaction effect (F=1.330, p=0.048). Both the high dose and the low dose of the BZT analog produced significant increase and decrease, respectively, from control values at several time points along the session (Figure 1a). To examine the effects of cocaine, ANOVA was calculated with a one between-subjects factor, treatment, with two levels (0 and 15 mg/kg of cocaine). Cocaine treatment increased locomotor activity relative to baseline values (the first 60 min of activity of the general control group), as revealed post hoc analysis (F=12.30, p<0.0001).
Effects of AHN-1055 on locomotor activity and c-Fos expression in the mouse striatum and comparison with a reference dose of cocaine. Panel in (a) shows the time course of the effects induced by AHN-1055 (0, 1, 3, and 10 mg/kg) on locomotor activity over a 2 h period. The low dose of AHN-1055 decreased, whereas the high dose increased, locomotor activity at several time points along the activity curves (a), although variations in accumulated scores (mean distance travelled per unit time) did not reach statistical significance (b). Induction of c-Fos in different striatal regions after treatment with AHN-1055 compared with the reference dose of cocaine (c, d). Cocaine stimulated c-Fos expression in both ventral and dorsal striatum, whereas the BZT analog was effective only in the DLst. Digital photomicrographs of c-Fos expression in DLst after treatment with vehicle and the high dose of AHN-1055 are shown in (e). The schematic diagram in (e) depicts the striatal regions considered for quantification. (*) indicates significant differences (p=0.05) from control values. cc, corpus callosum. Scale bar 100 μm.
We next examined striatal c-Fos expression induced by AHN-1055 and cocaine exposure. The results indicated that the high dose of AHN-1055 increased c-Fos activation in the DLst compared with control values (F=7.102, p=0.0006; p<0.05 by N–K test), but not in the other regions under investigation (Figure 1d and e). The low and moderate doses of AHN-1055 decreased the basal expression of c-Fos throughout, although not significantly so (Figure 1c and d). Cocaine treatment enhanced early-gene expression in the N Acc core (F=7.87, p=0.0004; p<0.05 by N–K test), DMst (F=9.691, p<0.0001; p<0.05 by N–K test), and DLst (F=7.102, p=0.0006; p<0.05 by N–K test), but not in the N Acc shell (F=2.087, p=0.1169) (Figure 1c, d and e). Therefore, these observations show clear differences between AHN-1055 and cocaine in their ability to stimulate of c-Fos protein in the striatum.
AHN-1055 Differs from D-amphetamine and Nomifensine in the Modulation Cocaine-Induced Locomotion and Stereotypy
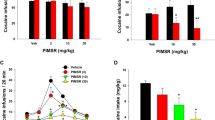
To gain insight into the possible modulatory effects of AHN-1055 on cocaine-stimulated motor activity, we carried out interaction assays in which AHN-1055 was given before cocaine administration. For comparison, we used a dopamine releaser, D-amphetamine, and a DAT inhibitor, nomifensine, as reference compounds. These drugs were administered before cocaine treatment, as done with AHN-1055. Figure 2 summarizes the findings. For the locomotor activity data, ANOVAs were performed with two between-subjects variables, pretreatment, with two levels (saline/AHN-1055, saline/D-amphetamine or saline/nomifensine), and posttreatment, with two levels (saline/cocaine), and two within-subjects variables, session, with five levels (5 days of treatments) and time, with four levels (four bins of 5 min each). In the AHN-1055 experiment, ANOVA revealed a significant interaction pretreatment × posttreatment (F=5.602, p=0.027), indicating that the effect of cocaine depended on earlier exposure to AHN-1055 (Figure 2a and b). Exploration of this interaction effect with post hoc tests indicated that AHN-1055 significantly increased locomotor activity compared with baseline values (p<0.01 by N–K test), and that the BZT analog significantly attenuated cocaine-induced hyperlocomotion (p< 0.05 by N–K test). When D-amphetamine was administered as a pretreatment, the interaction pretreatment × posttreatment was not significant (F=3.252, p=0.085) (Figure 2d and e). When combined with cocaine, the effects of nomifensine were similar to those evoked by pretreatment with the BZT analog. Nomifensine stimulated locomotor activity significantly, although to a lesser extent than cocaine, and attenuated cocaine-induced hyperlocomotion (Figure 2g). The ANOVA showed a significant interaction pretreatment × posttreatment (F=25.732, p=0.0001). However, contrary to what AHN-1055 produced, the stimulant effects of nomifensine waned with repeated exposure (Figure 2h).
Effects of AHN-1055 (10 mg/kg), D-amphetamine (4 mg/kg), and nomifensine (20 mg/kg) on cocaine-stimulated locomotor activity and motor stereotypy. When administered as a pretreatment, AHN-1055 reduced cocaine-induced hyperactivity (a, b) without potentiating the expression of stereotyped behaviors (c). By contrast, D-amphetamine showed more effectiveness that the BZT analog at stimulating locomotor activity (d, e) and enhanced motor stereotypy evoked by cocaine treatment (f) without decreasing cocaine-induced locomotion (d, e). Nomifensive decreased cocaine-stimulated locomotor activity (g, h) but produced strong stereotypies after repeated administration (i). The scores for the five daily sessions are shown in (a, d, and g) and the time course of effects in (b, e, and h). (*) indicates significant differences (p=0.05) from controls. (#) indicates significant differences (p=0.05) from cocaine values.
It was important at that point to determine the extent to which the effects of the pretreatments on cocaine-stimulated locomotor activity were affected by the expression of dopamine-dependent stereotypies. To that effect, we quantified such behaviors during the locomotor activity assays. In all cases, we carried out ANOVA on the ranked data with a factorial variable, treatment, with four levels (combinations of pretreatments with cocaine), and a repeated measure variable, session, with five levels (5 days of treatments). The analysis of motor stereotypy revealed that pretreatment with AHN-1055 did not potentiate cocaine-induced stereotypies, which emerged progressively with repeated cocaine administration (F=6.572, p=0.002; p<0.05 by G–H test) (Figure 2c). In contrast to AHN-1055, D-amphetamine enhanced motor stereotypies elicited by cocaine (F=6.646, p=0.002; p<0.05 by G–H test) (Figure 2f). Such an increase did not result from qualitative differences in the behavior exhibited by mice treated with the combination of D-amphetamine and cocaine. Instead, there was a generalized increment in the frequency and duration of repetitive behaviors, including mostly compulsive checking and stereotyped sniffing. We did quantify circling behavior (complete 360° turns usually followed by gnawing of the hind paws), but this behavior was not consistently induced by any of the drug treatments (data not shown). Although the effects of nomifensine resembled those of AHN-1055 in the initial sessions, similarly reducing cocaine-induced locomotor stimulation without increasing stereotyped behavior, repeated administration of nomifensine gave rise to sensitized, persistent head movements, and jerky body movements of high intensity (F=90.577, p=0.0001; p<0.05 by G–H test) (Figure 2i). Thus, the effects of AHN-1055 and nomifensine were clearly different with regards to the induction of motor stereotypy.
It is noteworthy that in the case of D-amphetamine, given alone or in combination with cocaine, the induction of intense motor stereotypies did not seem to attenuate the expression of hyperlocomotion, which did not decrease with repeated exposure (Figure 2e). In fact, the behavior of several animals treated with D-amphetamine and cocaine combined could be best described as ‘stereotyped locomotion’, a form of stimulant-induced hyperactivity characterized by repetitive selection of running paths (Bonasera et al, 2008). Upon repeated treatment with nomifensine, however, motor behaviors became more focused and spatially confined, and locomotor activity seemed to decrease gradually as a result (Figure 2h).
AHN-1055 Blocks Cocaine-Induce CPP and Neuronal Activation in the Striatum
We next studied the extent to which AHN-1055 treatment was able to influence cocaine-induced conditioned reward in the CPP paradigm. In parallel, we studied changes in locomotor activity and striatal early-gene expression in mice that were conditioned but killed after the last-conditioning session. The results of the behavioral and early-gene assays are depicted in Figures 3 and 4. For the CPP experiment, ANOVA was carried out with two between-subjects variables, pretreatment, with four levels (0, 1, 3, and 10 mg/kg AHN-1055), and posttreatment, with two levels (saline/cocaine) and one within-subjects variable, conditioning, with two levels (preconditioning and postconditioning). ANOVA indicated a significant high order effect pretreatment × posttreatment × conditioning (F=2.943, p=0.0376). Although there were no differences between the experimental groups in the preconditioning ratios, post hoc analysis of the data showed that cocaine exposure produced significant CPP. By contrast, AHN-1055 did not produce CPP, or place aversion, at any dose. Most remarkably, pretreatment with AHN-1055 dose-dependently blocked cocaine-induced CPP (Figure 3b).
Effects of AHN-1055 (0, 1, 3, and 10 mg/kg) on locomotor activity and place preference and interactions with cocaine. Conditioning effects in the CPP procedure are shown in (a). AHN-1055 failed to elicit conditioning at any of the doses evaluated. When given before cocaine exposure, the analog dose-dependently blocked cocaine-induced CPP. Overall mean distance travelled during the drug conditioning sessions is shown in (b). The low dose of AHN-1055 induced inhibitory effects on locomotor activity, but the high dose was without effect over the 4-day exposure period. AHN-1055 significantly reduced cocaine-stimulated locomotor activity. (*) indicates significant differences (p=0.05) from control values and (#) indicates significant deviations (p=0.05) from cocaine values. DP/NDP, drug paired/non drug paired.
Interactions of AHN-1055 (0, 1, 3, and 10 mg/kg) with cocaine on c-Fos induction in conditioned mice. AHN-1055 did not increase c-Fos expression in any of the striatal regions investigated. However, the BZT analog prevented cocaine-induced c-Fos expression in the N Acc core, N Acc shell and DMst (a, b). Digital photomicrographs of c-Fos expression in the ventral striatum after treatment with vehicle, cocaine and the combination of the high dose of AHN-1055 with cocaine are shown in (c). (*) indicates significant differences (p=0.05) from control values and (#) indicates significant deviations (p=0.05) from cocaine values. ac, anterior commissure. Scale bar 100 μm.
We next investigated locomotor activity and c-Fos expression in mice that were killed after the last-conditioning session. ANOVA was calculated with two between-subjects variables, pretreatment, with four levels (0, 1, 3, and 10 mg/kg AHN-1055), and posttreatment, with two levels (saline/cocaine) and one within-subjects variable, session, with four levels (4 days of drug conditioning). The locomotor activity assays confirmed our earlier data indicating that AHN-1055 attenuated the locomotor stimulation induced by cocaine. The results showed a significant interaction pretreatment × posttreatment (F=3.221, p=0.033), indicating that the effect of cocaine was dependent on earlier exposure to AHN-1055. The high dose of the AHN-1055 inhibited the locomotor activity evoked by cocaine (p<0.01 by N–K test) (Figure 3a). The suppressing effects of the BZT analog on cocaine-induced locomotor activity were homogeneous across sessions. When we examined the data averaged for the four sessions, we observed a significant inhibitory effect of the low dose of AHN-1055 (p<0.05 by N–K test), which was consistent through the 4 days of administration. The high dose was stimulant during the first session, but the mice developed tolerance to the activating effects of the BZT analog (data not shown). This finding is at variance with the results obtained in the earlier experiment in which high doses of AHN-1055 did not produce tolerance when administered daily, instead of given on alternate days.
We sought to determine whether the early-gene activation in the striatum correlated with the behavioral effects observed, both in the locomotor and CPP assays. Figure 4 shows the results. After 4 days of treatment, cocaine enhanced the expression of c-Fos in the N Acc core (F=10.90, p=0.0001; p<0.05 by N–K test), N Acc shell (F=6.068, p=0.0002; p<0.05 by N–K test), DMst (F=7.58, p<0.0001; p<0.05 by N–K test), but not in the DLst (F=0.9055, p=0.515), whereas treatment with AHN-1055 alone was without effect at any dose in any of the regions studied (Figure 4a, b and c). It is noted that the BZT analog prevented the induction of c-Fos in the same regions where cocaine effectively produced significant elevations.
DISCUSSION
The design and synthesis of N-substituted BZT analogs has provided new tools to explore the functional correlates of DAT activity. As a result, new avenues have been opened for reducing the adverse effects caused by the administration of cocaine and other stimulants with functional activity at the DAT. These experiments provide an ample set of novel behavioral observations that characterize the behavioral and neurochemical effects of the BZT analog, AHN-1055, and its interactions with cocaine in mice. Albeit AHN-1055 and parent compounds were first synthesized more than a decade ago, their potential as pharmacotherapies for cocaine addiction has not been sufficiently investigated in preclinical models. The data presented showed by means of complementary experiments that the BZT analog, despite its ability to bind with high affinity to the DAT, does not behave as a classical psychomotor stimulant and, most notably, does attenuate, or completely prevents, the activating effects of cocaine on striatal early-gene expression as well as some clinically relevant behavioral correlates of cocaine administration, including locomotor activity and conditioned reward.
We first studied the effects of AHN-1055 on locomotor activity and c-Fos expression in the striatum. AHN-1055 displayed inhibitory effects on locomotor activity at a low dose and stimulant effects at a high dose. The paradoxical effects of AHN-1055 observed in these experiments are consistent with earlier studies in the rat (Li et al, 2005). The pharmacological actions responsible for the inhibitory effect of the BZT analog are unclear. One possibility is that low doses of AHN-1055 produce weak elevations in extracellular dopamine concentrations, which preferentially stimulate dopamine autoreceptors, thereby producing a net decrease in the dopamine transmission. Alternatively, threshold elevations of extracellular dopamine could activate D2/D3-like postsynaptic receptors, whose selective stimulation has been associated with motor inhibition (Canales and Iversen, 1998; Canales and Iversen, 2000). Most remarkably, the biphasic pattern of locomotor activity observed after treatment with AHN-1055 is unlike that observed after administration of classical psychomotor stimulants, such as cocaine and D-amphetamine.
After acute administration, the paradoxical biphasic dose–response curve displayed by the BZT analog in the locomotor activity assays was paralleled by biphasic changes in early-gene activation in the striatum. AHN-1055 induced significant elevations in c-Fos protein expression in the DLst at the high dose, whereas evoking anatomically distributed but marginal downshifts at low and moderate doses. Again, this pattern of striatal activation induced by the BZT analog differed from that of cocaine, which evoked potent effects on early-gene expression in the ventral and dorsal striatal domains, as reported earlier (Canales and Graybiel, 2000; Canales, 2005). Other psychomotor stimulants, including D-amphetamine and methylphenidate, also diverged from AHN-1055 with regard to this dorsal–ventral selectivity (Moratalla et al, 1992; Robertson and Jian, 1995; Chase et al, 2005). The effects evoked by AHN-1055 on early-gene expression in the DLst could be attributed not only to the dopaminergic activity of the BZT analog but also to its anticholinergic profile, as other muscarinic receptor antagonists, including atropine and scopolamine, induced c-Fos protein in this striatal sector (Bernard et al, 1993).
In the interaction experiments performed with AHN-1055, D-amphetamine, and nomifensine, the BZT analog induced mild but significant stimulant effects on locomotor activity at the high dose. In the CPP experiments, locomotor stimulation was apparent after acute treatment with AHN-1055 but the stimulant effect decreased with repeated exposure. It should be noted that no such tolerance to the stimulant effects of AHN-1055 was observed when the BZT analog was administered daily in the interactions assays. AHN-1055 is a long-acting compound, exhibiting elevated plasma and brain concentrations over prolonged periods of time, which exceed 24 h in rat assays (Raje et al, 2003). It is probable that brain accumulation of the BZT analog after daily administration counteracted the expression of tolerance to the stimulant effects of the drug, although further experiments are required to test this hypothesis. An important result that emerged from the interaction assays is that AHN-1055 significantly attenuated cocaine-induced locomotor activity. Such property has been shown earlier for another BZT derivative, JHW007 (Desai et al, 2005b). Clearly, AHN-1055 could have potentiated the effects of cocaine because the level of locomotion evoked by the reference dose of cocaine was far from ceiling levels, as evidenced by the effects induced by both D-amphetamine treatment and combined treatment with D-amphetamine and cocaine. Further, the attenuation produced by the BZT derivative did not result from an enhancement of seemingly competitive behaviors, such as stereotypy. By contrast to the effects of the BZT analog, D-amphetamine increased cocaine-induced locomotor activity when administered as a pretreatment. Combined D-amphetamine and cocaine treatment produced both robust locomotion and stereotyped behavior. This was largely caused by the induction of route-tracing stereotypies, which are a correlate of incremented striatal activity in mice (Bonasera et al, 2008). Critically, the BZT analog also differed from the DAT inhibitor nomifensine, which attenuated cocaine-induced locomotor activity but induced by itself intense stereotypies after repeated exposure. Therefore, these data provide a clear behavioral demonstration that AHN-1055 differs from a prototypical psychomotor stimulant drug, D-amphetamine, and from another DAT inhibitor, nomifensine.
Having found that AHN-1055 exerted substantial antagonistic actions on cocaine-stimulated locomotor behavior, we asked whether the BZT analog could influence the subjective effects of cocaine, as measured in the place conditioning procedure, as well as the activation of brain circuits involved in drug-induced reward. Activation of telencephalic circuits spanning through the N Acc has been consistently associated with reward-related learning and drug-associated plasticity (Carelli, 2002; Ikemoto, 2007; Robinson and Kolb, 2004). The results showed that the BZT analog did not produce CPP when given 1 h before conditioning. This finding confirms and extends earlier observations indicating that AHN-1055 failed to induce CPP when administered at varying time points ranging from 0 to 90 min before conditioning, although within a dose range lower than that used in these experiments (Li et al, 2005). In line with these behavioral observations, our neurochemical assays showed that the BZT analog did not produce significant variations in c-Fos inducibility in any of the striatal regions after repeated administration and conditioning. Even the effects observed in the DLst after acute administration of AHN-1055 waned with repeated treatment. This neuroadaptation, which might reflect compensatory changes in response to sustained elevations in dopamine concentrations, closely paralleled the lack of stimulatory effects of AHN-1055 on locomotor activity after repeated exposure. Conversely, repeated cocaine exposure and place conditioning provoked potent effects on early-gene expression in the core and shell sectors of the N Acc and DMst. It is noteworthy that acute cocaine treatment did not elevate c-Fos levels in the shell sector of the N acc, but the early-gene response did sensitize with repeated exposure, as earlier shown in rats (Brenhouse and Stellar, 2006). A novel finding of these experiments was that pretreatment with the BZT analog blocked cocaine-stimulated CPP. It is noted that AHN-1055 also prevented in cocaine-conditioned mice the induction of early-gene activation in the N Acc and DMst, a finding that provides insight into the neural mechanisms underlying the ability of AHN-1055 to largely suppress the unconditioned and conditioned effects of cocaine.
These findings are best interpreted in the context of recent comparative molecular modelling studies. It has long been postulated that if cocaine-induced inhibition of dopamine transport resulted from allosteric modulation of the DAT, then it would be formally possible to design new molecules that might block cocaine's actions without affecting dopamine transport directly. Studies involving homology modelling and molecular simulation suggested that dopamine binds to the DAT in a hydrophobic pocket buried between transmembrane domains 1, 3, 6, and 8 (Huang and Zhan, 2007; Beuming et al, 2008). Using multiple docking approaches and mutagenesis, psychostimulant recognition sites for cocaine and D-amphetamine have been shown to extensively overlap with that of the endogenous substrate, dopamine (Indarte et al, 2008; Beuming et al, 2008). Therefore, the non-allosteric nature of cocaine's binding to the DAT renders the design of cocaine antagonists, which do not function as uptake inhibitors extremely troublesome. Interestingly, BZT derivatives, including JHW007 and MFZ 2–71, also bind in the vestibule at a site overlapping with that of dopamine (Beuming et al, 2008). In the light of such molecular findings, the most parsimonious explanation for the current data is that treatment with AHN-1055 diminished the ability of cocaine to induce neurochemical and behavioral effects through direct competition for a binding site at the DAT. Further studies should be performed to assess the contribution of muscarinic M1 receptors to cocaine-related neurochemical and behavioral effects, although our data with other BZT analogs with very low affinity for muscarinic M1 receptors suggest that their activity as DAT blockers is sufficient to reproduce the antagonistic behavioral actions of AHN-1055 (unpublished observations), in agreement with some earlier indications (Desai et al, 2005b).
In summary, these findings showed that AHN-1055, a BZT analog that exhibited weak pharmacological effects in early-gene, locomotor activity, and CPP assays, displayed marked antagonistic actions against cocaine. These observations provide support for further investigation of candidate medications that share a diphenylmethyl moiety, including BZT analogs, such as AHN-1055, modafinil (Dackis et al, 2005), and GBR series compounds (Rothman et al, 2008). Most notably, these findings highlight the possibility of designing novel compounds with affinity for the DAT which, by virtue of their differential pharmacokinetic/dynamic profile and mode of interaction with the DAT, might effectively antagonize the neuronal and behavioral actions of cocaine, even if they function as dopamine transport inhibitors.
DISCLOSURE/CONFLICT OF INTEREST
The author(s) declare that, except for income received from our primary employers, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH et al (1997a). Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40: 4329–4339.
Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH et al (1997b). Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40: 4329–4339.
Bernard V, Dumartin B, Lamy E, Bloch B (1993). Fos immunoreactivity after stimulation or inhibition of muscarinic receptors indicates anatomical specificity for cholinergic control of striatal efferent neurons and cortical neurons in the rat. Eur J Neurosci 5: 1218–1225.
Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K et al (2008). The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11: 780–789.
Bonasera SJ, Schenk AK, Luxenberg EJ, Tecott LH (2008). A novel method for automatic quantification of psychostimulant-evoked route-tracing stereotypy: application to Mus musculus. Psychopharmacology (Berl) 196: 591–602.
Brenhouse HC, Stellar JR (2006). c-Fos and deltaFosB expression are differentially altered in distinct subregions of the nucleus accumbens shell in cocaine-sensitized rats. Neuroscience 137: 773–780.
Canales JJ (2005). Intermittent cortical stimulation evokes sensitization to cocaine and enduring changes in matrix and striosome neuron responsiveness. Synapse 57: 56–60.
Canales JJ, Gilmour G, Iversen SD (2000). The role of nigral and thalamic output pathways in the expression of oral stereotypies induced by amphetamine injections into the striatum. Brain Res 856: 176–183.
Canales JJ, Graybiel AM (2000). A measure of striatal function predicts motor stereotypy. Nat Neurosci 3: 377–383.
Canales JJ, Iversen SD (1998). Behavioural topography in the striatum: differential effects of quinpirole and D-amphetamine microinjections. Eur J Pharmacol 362: 111–119.
Canales JJ, Iversen SD (2000). Dynamic dopamine receptor interactions in the core and shell of nucleus accumbens differentially coordinate the expression of unconditioned motor behaviors. Synapse 36: 297–306.
Carelli RM (2002). The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev 1: 281–296.
Carrigan KA, Dykstra LA (2007). Behavioral effects of morphine and cocaine in M1 muscarinic acetylcholine receptor-deficient mice. Psychopharmacology (Berl) 191: 985–993.
Chase TD, Carrey N, Brown RE, Wilkinson M (2005). Methylphenidate regulates c-fos and fosB expression in multiple regions of the immature rat brain. Brain Res Dev Brain Res 156: 1–12.
Chen N, Reith ME (2007). Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J Neurochem 101: 377–388.
Chen N, Zhen J, Reith ME (2004). Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR12909 and benztropine as opposed to cocaine. J Neurochem 89: 853–864.
Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O′Brien CP (2005). A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 30: 205–211.
Dar DE, Mayo C, Uhl GR (2005). The interaction of methylphenidate and benztropine with the dopamine transporter is different than other substrates and ligands. Biochem Pharmacol 70: 461–469.
Desai RI, Kopajtic TA, French D, Newman AH, Katz JL (2005a). Relationship between. J Pharmacol Exp Ther 315: 397–404.
Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL (2005b). Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25: 1889–1893.
Dutta AK, Zhang S, Kolhatkar R, Reith ME (2003). Dopamine transporter as target for drug development of cocaine dependence medications. Eur J Pharmacol 479: 93–106.
Grabowski J, Shearer J, Merrill J, Negus SS (2004). Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29: 1439–1464.
Hernandez-Rabaza V, Hontecillas-Prieto L, Velazquez-Sanchez C, Ferragud A, Perez-Villaba A, Arcusa A et al (2008). The hippocampal dentate gyrus is essential for generating contextual memories of fear and drug-induced reward. Neurobiol Learn Mem 90: 553–559.
Huang X, Zhan CG (2007). How dopamine transporter interacts with dopamine: Insights from molecular modeling and simulation. Biophys J 93: 3627–3639.
Ikemoto S (2007). Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev 56: 27–78.
Indarte M, Madura JD, Surratt CK (2008). Dopamine transporter comparative molecular modeling and binding site prediction using the LeuT(Aa) leucine transporter as a template. Proteins 70: 1033–1046.
Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S et al (2008). New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol 11: 425–438.
Katz JL, Agoston GE, Alling KL, Kline RH, Forster MJ, Woolverton WL et al (2001). Dopamine transporter binding without cocaine-like behavioral effects: synthesis and evaluation of benztropine analogs alone and in combination with cocaine in rodents. Psychopharmacology (Berl) 154: 362–374.
Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH (1999). Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther 288: 302–315.
Katz JL, Kopajtic TA, Agoston GE, Newman AH (2004). Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309: 650–660.
Kosten T, Owens SM (2005). Immunotherapy for the treatment of drug abuse. Pharmacol Ther 108: 76–85.
Li SM, Newman AH, Katz JL (2005). Place conditioning and locomotor effects of N-substituted, 4′,4′′-difluorobenztropine analogs in rats. J Pharmacol Exp Ther 313: 1223–1230.
Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K et al (2008). Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73: 813–823.
Moratalla R, Robertson HA, Graybiel AM (1992). Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J Neurosci 12: 2609–2622.
Raje S, Cao J, Newman AH, Gao H, Eddington ND (2003). Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther 307: 801–808.
Raje S, Cornish J, Newman AH, Cao J, Katz JL, Eddington ND (2005). Pharmacodynamic assessment of the benztropine analogues AHN-1055 and AHN-2005 using intracerebral microdialysis to evaluate brain dopamine levels and pharmacokinetic/pharmacodynamic modeling. Pharm Res 22: 603–612.
Robertson GS, Jian M (1995). D1 and D2 dopamine receptors differentially increase Fos-like immunoreactivity in accumbal projections to the ventral pallidum and midbrain. Neuroscience 64: 1019–1034.
Robinson TE, Kolb B (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 (Suppl 1): 33–46.
Rodriguez-Alarcon G, Canales JJ, Salvador A (2007). Rewarding effects of 3,4-methylenedioxymethamphetamine (‘Ecstasy’) in dominant and subordinate OF-1 mice in the place preference conditioning paradigm. Prog Neuropsychopharmacol Biol Psychiatry 31: 191–199.
Rothman RB (1990). High affinity dopamine reuptake inhibitors as potential cocaine antagonists: a strategy for drug development. Life Sci 46: L17–L21.
Rothman RB, Baumann MH, Prisinzano TE, Newman AH (2008). Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol 75: 2–16.
Sofuoglu M, Kosten TR (2005). Novel approaches to the treatment of cocaine addiction. CNS Drugs 19: 13–25.
Tanda G, Ebbs A, Newman AH, Katz JL (2005). Effects of 4′-chloro-3 alpha-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther 313: 613–620.
Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH et al (2007). Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther 321: 334–344.
Tanda G, Katz JL (2007). Muscarinic preferential M(1) receptor antagonists enhance the discriminative-stimulus effects of cocaine in rats. Pharmacol Biochem Behav 87: 400–404.
Ukairo OT, Bondi CD, Newman AH, Kulkarni SS, Kozikowski AP, Pan S et al (2005). Recognition of benztropine by the dopamine transporter (DAT) differs from that of the classical dopamine uptake inhibitors cocaine, methylphenidate, and mazindol as a function of a DAT transmembrane 1 aspartic acid residue. J Pharmacol Exp Ther 314: 575–583.
Vaughan RA, Agoston GE, Lever JR, Newman AH (1999). Differential binding of tropane-based photoaffinity ligands on the dopamine transporter. J Neurosci 19: 630–636.
Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D et al (2000). Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci 67: 1507–1515.
Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J et al (1996). Relationship between psychostimulant-induced ‘high’ and dopamine transporter occupancy. Proc Natl Acad Sci USA 93: 10388–10392.
Conover WJ, Iman RL (1981). Rank transformations as a bridge between parametric and non-parametric statistics. Am Stat 35: 124–129.
Paxinos G, Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates. Academic Press: New York.
Acknowledgements
This work was supported by grants to JJC from Plan Nacional de Biomedicina (Ministerio de Ciencia e Innovación), Plan Nacional Sobre Drogas (Ministerio de Sanidad y Consumo), Red de Trastornos Adictivos (RETICS, Instituto de Salud Carlos III), and FEPAD (Fundación para el Estudio y Prevención de las Adicciones y Drogodependencias, Generalitat Valenciana). CV-S is in receipt of a graduate student contract from Red de Trastornos Adictivos (RETICS, Instituto de Salud Carlos III). We thank Alexandra Arcusa for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velázquez-Sánchez, C., Ferragud, A., Hernández-Rabaza, V. et al. The Dopamine Uptake Inhibitor 3α-[bis(4′-fluorophenyl)metoxy]-tropane Reduces Cocaine-Induced Early-Gene Expression, Locomotor Activity, and Conditioned Reward. Neuropsychopharmacol 34, 2497–2507 (2009). https://doi.org/10.1038/npp.2009.78
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2009.78
Keywords
This article is cited by
-
Effects of benztropine analogs on delay discounting in rats
Psychopharmacology (2020)
-
Behavioral economic analysis of the effects of N-substituted benztropine analogs on cocaine self-administration in rats
Psychopharmacology (2018)
-
The stereotypy-inducing effects of N-substituted benztropine analogs alone and in combination with cocaine do not account for their blockade of cocaine self-administration
Psychopharmacology (2013)