Abstract
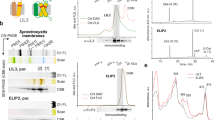
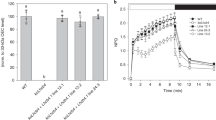
Oxygenic photoautotrophs require mechanisms for rapidly matching the level of chlorophyll excited states from light harvesting with the rate of electron transport from water to carbon dioxide. These photoprotective reactions prevent formation of reactive excited states and photoinhibition. The fastest response to excess illumination is the so-called non-photochemical quenching which, in higher plants, requires the luminal pH sensor PsbS and other yet unidentified components of the photosystem II antenna. Both trimeric light-harvesting complex II (LHCII) and monomeric LHC proteins have been indicated as site(s) of the heat-dissipative reactions. Different mechanisms have been proposed: energy transfer to a lutein quencher in trimers, formation of a zeaxanthin radical cation in monomers. Here, we report on the construction of a mutant lacking all monomeric LHC proteins but retaining LHCII trimers. Its non-photochemical quenching induction rate was substantially slower with respect to the wild type. A carotenoid radical cation signal was detected in the wild type, although it was lost in the mutant. We conclude that non-photochemical quenching is catalysed by two independent mechanisms, with the fastest activated response catalysed within monomeric LHC proteins depending on both zeaxanthin and lutein and on the formation of a radical cation. Trimeric LHCII was responsible for the slowly activated quenching component whereas inclusion in supercomplexes was not required. This latter activity does not depend on lutein nor on charge transfer events, whereas zeaxanthin was essential.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vass, I. et al. Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc. Natl Acad. Sci. USA 89, 1408–1412 (1992).
Niyogi, K. K. & Truong, T. B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 16, 307–314 (2013).
de Bianchi, S., Ballottari, M., Dall'Osto, L. & Bassi, R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem. Soc. Trans. 38, 651–660 (2010).
Horton, P., Ruban, A. V. & Walters, R. G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 (1996).
Cazzaniga, S., Dall'Osto, L., Kong, S. G., Wada, M. & Bassi, R. Interaction between avoidance of photon absorption, excess energy dissipation and zeaxanthin synthesis against photoxidative stress in Arabidopsis. Plant J. 76, 568–579 (2013).
Nilkens, M. et al. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta. 1797, 466–475 (2010).
Li, X. P. et al. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279, 22866–22874 (2004).
Dominici, P. et al. Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J. Biol. Chem. 277, 22750–22758 (2002).
Fan, M. et al. Crystal structures of the PsbS protein essential for photoprotection in plants. Nat. Struct. Mol. Biol. 22, 729–735 (2015).
Suga, M. et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517, 99–103 (2015).
Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 236–240 (1999).
Wei, X. et al. Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature 534, 69–74 (2016).
Kouril, R., Wientjes, E., Bultema, J. B., Croce, R. & Boekema, E. J. High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. BBA-Bioenergetics 1827, 411–419 (2013).
Betterle, N. et al. Light-induced dissociation of an antenna hetero-oligomer is needed for non-photochemical quenching induction. J. Biol. Chem. 284, 15255–15266 (2009).
Niyogi, K. K. et al. Photoprotection in a zeaxanthin- and lutein-deficient double mutant of Arabidopsis. Photosynth. Res. 67, 139–145 (2001).
Havaux, M., Dall'Osto, L. & Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 145, 1506–1520 (2007).
Belgio, E., Johnson, M. P., Juric, S. & Ruban, A. V. Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime-both the maximum and the nonphotochemically quenched. Biophys J. 102, 2761–2771 (2012).
Ruban, A. V., Walters, R. G. & Horton, P. The molecular mechanism of the control of excitation energy dissipation in chloroplast membranes—inhibition of deltapH- dependent quenching of chlorophyll fluorescence by dicyclohexylcarbodiimide. FEBS Lett. 309, 175–179 (1992).
Ruban, A. V. et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578 (2007).
Niyogi, K. K., Grossman, A. R. & Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121–1134 (1998).
Muller, M. G. et al. Singlet energy dissipation in the photosystem II light-harvesting complex does not involve energy transfer to carotenoids. ChemPhysChem 11, 1289–1296 (2010).
Wahadoszamen, M., Berera, R., Ara, A. M., Romero, E. & van Grondelle, R. Identification of two emitting sites in the dissipative state of the major light harvesting antenna. Phys. Chem. Chem. Phys. 14, 759–766 (2012).
Bode, S. et al. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl Acad. Sci. USA 106, 12311–12316 (2009).
Holt, N. E. et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307, 433–436 (2005).
Avenson, T. J. et al. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J. Biol. Chem. 283, 3550–3558 (2008).
Ahn, T. K. et al. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320, 794–797 (2008).
Barros, T., Royant, A., Standfuss, J., Dreuw, A. & Kuhlbrandt, W. Crystal structure of plant light-harvesting complex shows the active, energy-transmitting state. EMBO J. 28, 298–306 (2009).
Avenson, T. J. et al. Lutein can act as a switchable charge transfer quencher in the CP26 light-harvesting complex. J. Biol. Chem. 284, 2830–2835 (2009).
de Bianchi, S., Dall'Osto, L., Tognon, G., Morosinotto, T. & Bassi, R. Minor antenna proteins CP24 and CP26 affect the interactions between photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 20, 1012–1028 (2008).
de Bianchi, S. et al. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell 23, 2659–2679 (2011).
Dall'Osto, L., Unlu, C., Cazzaniga, S. & van Amerongen, H. Disturbed excitation energy transfer in Arabidopsis thaliana mutants lacking minor antenna complexes of photosystem II. Biochim. Biophys. Acta 1837, 1981–1988 (2014).
Baker, N. R. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113 (2008).
Johnson, G. N., Young, A. J. & Horton, P. Activation of non-photochemical quenching in thylakoids and leaves. Planta 194, 550–556 (1994).
Finazzi, G. et al. A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc. Natl Acad. Sci. USA 101, 12375–12380 (2004).
Pogson, B. J., Niyogi, K. K., Bjorkman, O. & DellaPenna, D. Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc. Natl Acad. Sci. USA 95, 13324–13329 (1998).
Horton, P. et al. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll-protein complex. FEBS Lett. 292, 1–4 (1991).
Lambrev, P. H., Nilkens, M., Miloslavina, Y., Jahns, P. & Holzwarth, A. R. Kinetic and spectral resolution of multiple nonphotochemical quenching components in Arabidopsis leaves. Plant Physiol. 152, 1611–1624 (2010).
Holzwarth, A. R., Miloslavina, Y., Nilkens, M. & Jahns, P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 483, 262–267 (2009).
Li, X. P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000).
Caffarri, S., Frigerio, S., Olivieri, E., Righetti, P. G. & Bassi, R. Differential accumulation of Lhcb gene products in thylakoid membranes of Zea mays plants grown under contrasting light and temperature conditions. Proteomics 5, 758–768 (2005).
Briantais, J.-M., Hodges, M. & Moya, I. in Progress in Photosynthesis Research II (ed. J. Biggins ) 705–708 (Nijhoff Publishers, 1987).
Pietrzykowska, M. et al. The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 26, 3646–3660 (2014).
Leoni, C. et al. Very rapid phosphorylation kinetics suggest a unique role for Lhcb2 during state transitions in Arabidopsis. Plant J. 76, 236–246 (2013).
Damkjaer, J. et al. The photosystem II light-harvesting protein Lhcb3 affects the macrostructure of photosystem II and the rate of state transitions in Arabidopsis. Plant Cell 21, 3245–3256 (2009).
Li, Z. et al. Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 21, 1798–1812 (2009).
Lambrev, P. H. et al. Functional domain size in aggregates of light-harvesting complex II and thylakoid membranes. BBA-Bioenergetics 1807, 1022–1031 (2011).
Miloslavina, Y. et al. Far-red fluorescence: a direct spectroscopic marker for LHCII oligomer formation in non-photochemical quenching. FEBS Lett. 582, 3625–3631 (2008).
Wilk, L., Grunwald, M., Liao, P. N., Walla, P. J. & Kuhlbrandt, W. Direct interaction of the major light-harvesting complex II and PsbS in nonphotochemical quenching. Proc. Natl Acad. Sci. USA 110, 5452–5456 (2013).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004).
Caffarri, S., Croce, R., Breton, J. & Bassi, R. The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J. Biol. Chem. 276, 35924–35933 (2001).
Weis, E. & Berry, J. A. Quantum efficiency of photosystem II in relation to energy dependent quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 894, 198–208 (1987).
Haniewicz, P. et al. Isolation of monomeric photosystem II that retains the subunit PsbS. Photosynth. Res. 118, 199–207 (2013).
Morosinotto, T., Baronio, R. & Bassi, R. Dynamics of chromophore binding to Lhc proteins in vivo and in vitro during operation of the xanthophyll cycle. J. Biol. Chem. 277, 36913–36920 (2002).
Walters, R. G., Ruban, A. V. & Horton, P. Identification of proton-active residues in a higher plant light-harvesting complex. Proc. Natl Acad. Sci. USA 93, 14204–14209 (1996).
Casazza, A. P., Tarantino, D. & Soave, C. Preparation and functional characterization of thylakoids from Arabidopsis thaliana. Photosynth. Res. 68, 175–180 (2001).
Gilmore, A. M. & Yamamoto, H. Y. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 96, 635–643 (1991).
Schägger, H. & von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166, 368–379 (1987).
Havaux, M., Dall'Osto, L., Cuine, S., Giuliano, G. & Bassi, R. The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J. Biol. Chem. 279, 13878–13888 (2004).
Augulis, R. & Zigmantas, D. Two-dimensional electronic spectroscopy with double modulation lock-in detection: enhancement of sensitivity and noise resistance. Opt. Express. 19, 13126–13133 (2011).
Acknowledgements
This work was supported by the EEC projects ACCLIPHOT (PITN-GA-2012-316427) and SE2B (675006–SE2B) to R.B. Work in Lund was supported by LaserLab Europe, the Swedish Research Council and the Knut and Alice Wallenberg Foundation. The work of K.K.N. and G.R.F. was supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DEAC02-05CH11231 and the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences under field work proposal 449B. L.D.'s work was supported by international mobility programme CooperInt 2011/2014, University of Verona.
Author information
Authors and Affiliations
Contributions
R.B., K.K.N. and G.R.F. conceived the work and designed the experiments. L.D., S.C. and M.B. performed all the experiments for the isolation of mutants, and their physiological and biochemical characterization. D.Z. coordinated and performed the transient absorption spectroscopy experiments. D.P. and K.Z. contributed to the time resolved analysis experiments. All of the authors contributed to writing the manuscript. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–6, Supplementary Table 1 and Supplementary References. (PDF 1038 kb)
Rights and permissions
About this article
Cite this article
Dall'Osto, L., Cazzaniga, S., Bressan, M. et al. Two mechanisms for dissipation of excess light in monomeric and trimeric light-harvesting complexes. Nature Plants 3, 17033 (2017). https://doi.org/10.1038/nplants.2017.33
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2017.33
This article is cited by
-
The nature of carotenoid S* state and its role in the nonphotochemical quenching of plants
Nature Communications (2024)
-
Excitation transfer and quenching in photosystem II, enlightened by carotenoid triplet state in leaves
Photosynthesis Research (2024)
-
Cryo-EM structures of LHCII in photo-active and photo-protecting states reveal allosteric regulation of light harvesting and excess energy dissipation
Nature Plants (2023)
-
Oxidative stability of lutein on exposure to varied extrinsic factors
Journal of Food Science and Technology (2023)
-
Recent progress in atomistic modeling of light-harvesting complexes: a mini review
Photosynthesis Research (2023)