Abstract
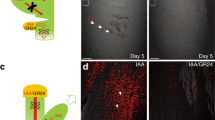
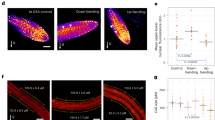
The phytohormone auxin induces or represses growth depending on its concentration and the underlying tissue type. However, it remains unknown how auxin signalling is modulated to allow tissues transiting between repression and promotion of growth. Here, we used apical hook development as a model for growth transitions in plants. A PIN-FORMED (PIN)-dependent intercellular auxin transport module defines an auxin maximum that is causal for growth repression during the formation of the apical hook. Our data illustrate that growth transition for apical hook opening is largely independent of this PIN module, but requires the PIN-LIKES (PILS) putative auxin carriers at the endoplasmic reticulum. PILS proteins reduce nuclear auxin signalling in the apical hook, leading to the de-repression of growth and the onset of hook opening. We also show that the phytochrome (phy) B-reliant light-signalling pathway directly regulates PILS gene activity, thereby enabling light perception to repress nuclear auxin signalling and to control growth. We propose a novel mechanism, in which PILS proteins allow external signals to alter tissue sensitivity to auxin, defining differential growth rates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
04 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41477-021-00924-y
References
Dharmasiri, N., Dharmasiri, S. & Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 435, 441–445 (2005).
Kepinski, S. & Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446–451 (2005).
Rosquete, M. R., Barbez, E. & Kleine-Vehn, J. Cellular auxin homeostasis: gatekeeping is housekeeping. Mol. Plant 5, 772–786 (2012).
Evans, M. L., Ishikawa, H. & Estelle, M. A. Responses of Arabidopsis roots to auxin studied with high temporal resolution: comparison of wild type and auxin-response mutants. Planta 194, 215–222 (1994).
Abbas, M., Alabadí, D. & Blázquez, M. A. Differential growth at the apical hook: all roads lead to auxin. Front Plant Sci. 4, 441 (2013).
Vandenbussche, F. et al. The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137, 597–606 (2010).
Žádníková, P. et al. Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137, 607–617 (2010).
Žádníková, P. et al. A model of differential growth-guided apical hook formation in plants. Plant Cell 28, 2464–2477 (2016).
Willige, B. C., Ogiso-Tanaka, E., Zourelidou, M. & Schwechheimer, C. WAG2 represses apical hook opening downstream from gibberellin and PHYTOCHROME INTERACTING FACTOR 5. Development 139, 4020–4028 (2012).
Rubinstein, B. Auxin and red light in the control of hypocotyl hook opening in beans. Plant Physiol. 48, 187–192 (1971).
Yu, Q. et al. Clathrin-mediated auxin efflux and maxima regulate hypocotyl hook formation and light-stimulated hook opening in Arabidopsis. Mol. Plant 9, 101–112 (2016).
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003).
Petrášek, J. et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–918 (2006).
Grieneisen, V. A., Xu, J., Marée, A. F., Hogeweg, P. & Scheres, B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449, 1008–1013 (2007).
Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008).
Won, C. et al. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 18518–18523 (2011).
Mashiguchi, K. et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl Acad. Sci. USA 108, 18512–18517 (2011).
Boer, D. R. et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 156, 577–589 (2014).
Barbez, E. et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485, 119–122 (2012).
Barbez, E. et al. Single-cell-based system to monitor carrier driven cellular auxin homeostasis. BMC Plant Biol. 13, 20 (2013).
Reed, J. W. & Chory, J. Mutational analyses of light-controlled seedling development in Arabidopsis. Semin. Cell Biol. 5, 327–334 (1994).
Friml, J. et al. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 426, 147–153 (2003).
Duek, P. D. & Fankhauser, C. bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 10, 51–54 (2005).
Vieten, A. et al. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132, 4521–4531 (2005).
de Wit, M. & Pierik, R. in Canopy Photosynthesis: From Basics to Applications (eds Hikosaka, K., Niinemets, Ü. & Anten, N. P. R. ) 171–186 (Springer, 2016).
Hornitschek, P. et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 71, 699–711 (2012).
Franklin, K. A. et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl Acad. Sci. USA 108, 20231–20235 (2011).
Hersch, M. et al. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl Acad. Sci. USA 111, 6515–6520 (2014).
Krishna Reddy, S. & Finlayson, S. A. Phytochrome B promotes branching in Arabidopsis by suppressing auxin signaling. Plant Physiol. 164, 1542–1550 (2014).
Fendrych, M., Leung, J. & Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. eLife 5, 297 (2016).
Reed, J. W., Nagatani, A., Elich, T. D., Fagan, M. & Chory, J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104, 1139–1149 (1994).
Marin, E. et al. Mir390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 22, 1104–1117 (2010).
Béziat, C., Kleine-Vehn, J. & Feraru, E. Histochemical staining of β-glucuronidase and its spatial quantification. Methods Mol. Biol. 1497, 73–80 (2017).
Gendrel, A.-V., Lippman, Z., Yordan, C., Colot, V. & Martienssen, R. A. Dependence of heterochromatic histone H3 methylation patterns on the Arabidopsis gene DDM1. Science 297, 1871–1873 (2002).
Kumar, S. V. et al. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245 (2012).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408 (2001).
Acknowledgements
We are grateful to P. Zadnikova and E. Benkova for introducing us to infrared-based time lapse imaging; D. Scheuring and F. Barbez for helping us establishing our infrared imaging station; C. Fankhauser, P. Wigge, J. Friml and A. Maizel for providing published material; E. Benkova, M. Geisler, U. Hammes and J.K.-V. group members for critical reading of the manuscript; J. Thacker for help with the manuscript; and the BOKU-VIBT Imaging Centre for access and expertise. This work was supported by the Vienna Research Group (VRG) programme of the Vienna Science and Technology Fund (WWTF), the Austrian Science Fund (FWF) (Projects: P26568-B16 and P26591-B16) and the European Research Council (ERC) (Starting Grant 639478-AuxinER) (to J.K.-V.) as well as the APART fellowship of the Austrian Academy of Sciences (ÖAW) (to D.L.).
Author information
Authors and Affiliations
Contributions
C.B. carried out most of the experiments; J.K.-V. and C.B. interpreted the results and designed experiments. E.B. cloned most constructs and contributed to the statistical analysis. M.I.F. genotyped pils loss-of-function mutant and crosses. D.L. and C.B. performed ChIP and qPCR experiments, J.K.-V. and C.B. wrote the manuscript. All authors saw and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–12, Supplementary Table 1. (PDF 7526 kb)
Rights and permissions
About this article
Cite this article
Béziat, C., Barbez, E., Feraru, M. et al. Light triggers PILS-dependent reduction in nuclear auxin signalling for growth transition. Nature Plants 3, 17105 (2017). https://doi.org/10.1038/nplants.2017.105
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2017.105
This article is cited by
-
Full-length transcriptome and metabolism revealed the difference of callus formation of tea cutting under white, red and blue light
Plant Growth Regulation (2023)
-
Molecular mechanisms to sense soil overlay and optimize emergence in plants
Journal of Plant Biochemistry and Biotechnology (2021)
-
Gene Characterization and Expression Analysis Reveal the Importance of Auxin Signaling in Bud Dormancy Regulation in Tea Plant
Journal of Plant Growth Regulation (2019)