Abstract
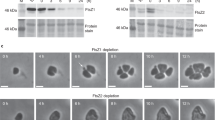
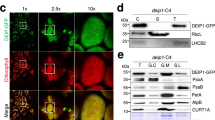
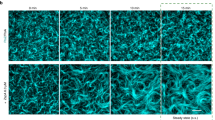
Chloroplast division is driven by a ring containing FtsZ1 and FtsZ2 proteins, which originated from bacterial FtsZ, a tubulin-like protein; however, mechanistic details of the chloroplast FtsZ ring remain unclear. Here, we report that FtsZ1 and FtsZ2 can heteropolymerize into a contractible ring ex vivo. Fluorescently labelled FtsZ1 and/or FtsZ2 formed single rings in cells of the yeast Pichia pastoris. Photobleaching experiments indicated that co-assembly of FtsZ1 and FtsZ2 imparts polarity to polymerization. Assembly of FtsZ chimaeras revealed that the protofilaments assemble via heteropolymerization of FtsZ2 and FtsZ1. Contraction of the ring was accompanied by an increase in the filament turnover rate. Our findings suggest that the evolutionary duplication of FtsZ in plants may have increased the mobility and kinetics of FtsZ ring dynamics in chloroplast division. Thus, the gene duplication and heteropolymerization of chloroplast FtsZs may represent convergent evolution with eukaryotic tubulin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jarvis, P. & López-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nature Rev. Mol. Cell Biol. 14, 787–802 (2013).
Gillham, N. W., Boynton, J. E. & Hauser, C. R. Translational regulation of gene expression in chloroplasts and mitochondria. Annu. Rev. Genet. 28, 71–93 (1994).
Yoshida, Y., Miyagishima, S. Y., Kuroiwa, H. & Kuroiwa, T. The plastid-dividing machinery: formation, constriction and fission. Curr. Opin. Plant Biol. 15, 714–721 (2012).
Osteryoung, K. W. & Pyke, K. A. Division and dynamic morphology of plastids. Annu. Rev. Plant Biol. 65, 443–472 (2014).
Kuroiwa, T. et al. Vesicle, mitochondrial, and plastid division machineries with emphasis on dynamin and electron-dense rings. Int. Rev. Cell Mol. Biol. 271, 97–152 (2008).
Miyagishima, S. Y. & Kabeya, Y. Chloroplast division: squeezing the photosynthetic captive. Curr. Opin. Microbiol. 13, 738–746 (2010).
Mori, T., Kuroiwa, H., Takahara, M., Miyagishima, S. Y. & Kuroiwa, T. Visualization of an FtsZ ring in chloroplasts of Lilium longiflorum leaves. Plant Cell Physiol. 42, 555–559 (2001).
Vitha, S., McAndrew, R. S. & Osteryoung, K. W. FtsZ ring formation at the chloroplast division site in plants. J. Cell Biol. 153, 111–120 (2001).
Kuroiwa, T. et al. The division apparatus of plastids and mitochondria. Int. Rev. Cytol. 181, 1–41 (1998).
Yoshida, Y. et al. Chloroplasts divide by contraction of a bundle of nanofilaments consisting of polyglucan. Science 329, 949–953 (2010).
Miyagishima, S. Y. et al. A plant-specific dynamin-related protein forms a ring at the chloroplast division site. Plant Cell Online 15, 655–665 (2003).
Gao, H., Kadirjan-Kalbach, D., Froehlich, J. E. & Osteryoung, K. W. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc. Natl Acad. Sci. USA 100, 4328–4333 (2003).
Hong, Z. et al. A unified nomenclature for Arabidopsis dynamin-related large GTPases based on homology and possible functions. Plant Mol. Biol. 53, 261–265 (2003).
Osteryoung, K. W. & Vierling, E. Conserved cell and organelle division. Nature 376, 473–474 (1995).
Takahara, M. et al. A putative mitochondrial ftsZ gene is present in the unicellular primitive red alga Cyanidioschyzon merolae. Mol. Gen. Genet. 264, 452–460 (2000).
Adams, D. W. & Errington, J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nature Rev. Microbiol. 7, 642–653 (2009).
Erickson, H. P., Anderson, D. E. & Osawa, M. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 74, 504–528 (2010).
Miyagishima, S. Y. et al. Two types of FtsZ proteins in mitochondria and red-lineage chloroplasts: the duplication of FtsZ is implicated in endosymbiosis. J. Mol. Evol. 58, 291–303 (2004).
TerBush, A. D., Yoshida, Y. & Osteryoung, K. W. FtsZ in chloroplast division: structure, function and evolution. Curr. Opin. Cell Biol. 25, 461–470 (2013).
Osteryoung, K. W., Stokes, K. D., Rutherford, S. M., Percival, A. L. & Lee, W. Y. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ. Plant Cell. 10, 1991–2004 (1998).
Ma, X. & Margolin, W. Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J. Bacteriol. 181, 7531–7544 (1999).
Maple, J., Aldridge, C. & Møller, S. G. Plastid division is mediated by combinatorial assembly of plastid division proteins. Plant J. 43, 811–823 (2005).
Schmitz, A. J., Glynn, J. M., Olson, B. J. S. C., Stokes, K. D. & Osteryoung, K. W. Arabidopsis FtsZ2-1 and FtsZ2-2 are functionally redundant, but FtsZ-based plastid division is not essential for chloroplast partitioning or plant growth and development. Mol. Plant 2, 1211–1222 (2009).
Strepp, R., Scholz, S., Kruse, S., Speth, V. & Reski, R. Plant nuclear gene knockout reveals a role in plastid division for the homolog of the bacterial cell division protein FtsZ, an ancestral tubulin. Proc. Natl Acad. Sci. USA 95, 4368–4373 (1998).
Yoshida, Y. et al. Isolated chloroplast division machinery can actively constrict after stretching. Science 313, 1435–1438 (2006).
Osawa, M., Anderson, D. E. & Erickson, H. P. Reconstitution of contractile FtsZ rings in liposomes. Science 320, 792–794 (2008).
Nogales, E., Wolf, S. G., Downing, K. H., Gray, R. A. & Pertsov, A. M. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 393, 199–203 (1998).
Nogales, E., Whittaker, M., Milligan, R. A., Downing, K. H. & Berkeley, L. High-resolution model of the microtubule. Cell 96, 79–88 (1999).
Löwe, J. & Amos, L. A. Crystal structure of the bacterial cell-division protein FtsZ. Nature 391, 203–206 (1998).
Oliva, M. A., Cordell, S. C. & Löwe, J. Structural insights into FtsZ protofilament formation. Nature Struct. Mol. Biol. 11, 1243–1250 (2004).
Sprague, B. L., Pego, R. L., Stavreva, D. A. & McNally, J. G. Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys. J. 86, 3473–3495 (2004).
Olson, B. J. S. C., Wang, Q. & Osteryoung, K. W. GTP-dependent heteropolymer formation and bundling of chloroplast FtsZ1 and FtsZ2. J. Biol. Chem. 285, 20634–20643 (2010).
Zhang, M., Chen, C., Froehlich, J. E., Terbush, A. D. & Osteryoung, K. W. Roles of Arabidopsis PARC6 in coordination of the chloroplast division complex and negative regulation of FtsZ assembly. Plant Physiol. 170, 250–262 (2015).
Glynn, J. M., Froehlich, J. E. & Osteryoung, K. W. Arabidopsis ARC6 coordinates the division machineries of the inner and outer chloroplast membranes through interaction with PDV2 in the intermembrane space. Plant Cell 20, 2460–2470 (2008).
Miyagishima, S. Y. Mechanism of plastid division: from a bacterium to an organelle. Plant Physiol. 155, 1533–1544 (2011).
TerBush, A. D. & Osteryoung, K. W. Distinct functions of chloroplast FtsZ1 and FtsZ2 in Z-ring structure and remodeling. J. Cell Biol. 199, 623–637 (2012).
Nogales, E., Downing, K. H., Amos, L. A. & Löwe, J. Tubulin and FtsZ form a distinct family of GTPases. Nature Struct. Biol. 5, 451–458 (1998).
Halavatyi, A. et al. A mathematical model of actin filament turnover for fitting FRAP data. Eur. Biophys. J. 39, 669–677 (2010).
Acknowledgements
We thank K. Porter for helpful discussions. This research was supported by a Human Frontier Science Program Long Term Fellowship (LT000356/2011-L to Y.Y.), a Japan Society for the Promotion of Science Postdoctoral Research Fellowship for Research Abroad (to Y.Y.) and the National Science Foundation (grant MCB-1121943 to K.W.O.).
Author information
Authors and Affiliations
Contributions
Y.Y. designed the research and experiments. Y.Y. and Y.M. performed experiments and analysed the results. Y.Y., Y.M., A.D.T. and K.W.O. discussed the results. Y.Y., Y.M., A.D.T. and K.W.O. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Material
Supplementary Methods, Supplementary References, Supplementary Figs 1-8 and Supplementary Tables 1 and 2. (PDF 1829 kb)
Rights and permissions
About this article
Cite this article
Yoshida, Y., Mogi, Y., TerBush, A. et al. Chloroplast FtsZ assembles into a contractible ring via tubulin-like heteropolymerization. Nature Plants 2, 16095 (2016). https://doi.org/10.1038/nplants.2016.95
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.95
This article is cited by
-
A mysterious cloak: the peptidoglycan layer of algal and plant plastids
Protoplasma (2023)
-
The cellular machineries responsible for the division of endosymbiotic organelles
Journal of Plant Research (2018)
-
ARC6-mediated Z ring-like structure formation of prokaryote-descended chloroplast FtsZ in Escherichia coli
Scientific Reports (2017)
-
Three rings for the evolution of plastid shape: a tale of land plant FtsZ
Protoplasma (2017)