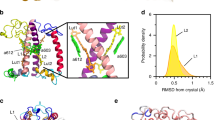
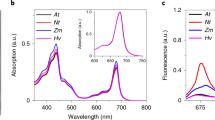
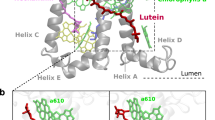
Abstract
The photosynthetic apparatus of green plants is well known for its extremely high efficiency that allows them to operate under dim light conditions. On the other hand, intense sunlight may result in overexcitation of the light-harvesting antenna and the formation of reactive compounds capable of ‘burning out’ the whole photosynthetic unit. Non-photochemical quenching is a self-regulatory mechanism utilized by green plants on a molecular level that allows them to safely dissipate the detrimental excess excitation energy as heat. Although it is believed to take place in the plant's major light-harvesting complexes (LHC) II, there is still no consensus regarding its molecular nature. To get more insight into its physical origin, we performed high-resolution time-resolved fluorescence measurements of LHCII trimers and their aggregates across a wide temperature range. Based on simulations of the excitation energy transfer in the LHCII aggregate, we associate the red-emitting state, having fluorescence maximum at ∼700 nm, with the partial mixing of excitonic and chlorophyll–chlorophyll charge transfer states. On the other hand, the quenched state has a totally different nature and is related to the incoherent excitation transfer to the short-lived carotenoid excited states. Our results also show that the required level of photoprotection in vivo can be achieved by a very subtle change in the number of LHCIIs switched to the quenched state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Croce, R. & van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nature Chem. Biol. 10, 492–501 (2014).
van Amerongen, H., Valkunas, L. & van Grondelle, R. Photosynthetic Excitons (World Scientific, 2000).
Holt, N. E. et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307, 433–436 (2005).
Pascal, A. A. et al. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436, 134–137 (2005).
Ruban, A. V. et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578 (2007).
Ahn, T. K. et al. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320, 794–797 (2008).
Ruban, A. V., Johnson, M. P. & Duffy, C. D. P. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta Bioenerg. 1817, 167–181 (2012).
Staleva, H. et al. Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nature Chem. Biol. 11, 287–291 (2015).
Wientjes, E., van Amerongen, H. & Croce, R. Quantum yield of charge separation in photosystem II: functional effect of changes in the antenna size upon light acclimation. J. Phys. Chem. B 117, 11200–11208 (2013).
Belgio, E. et al. Economic photoprotection in photosystem II that retains a complete light-harvesting system with slow energy traps. Nature Commun. 5, 4433 (2014).
Müller, M. G. et al. Singlet energy dissipation in the photosystem II light-harvesting complex does not involve energy transfer to carotenoids. ChemPhysChem 11, 1289–1296 (2010).
van Amerongen, H. & van Grondelle, R. Understanding the energy transfer function of LHCII, the major light-harvesting complex of green plants. J. Phys. Chem. B 105, 604–617 (2001).
Walla, P. J., Linden, P. A., Ohta, K. & Fleming, G. R. Excited-state kinetics of the carotenoid S1 state in LHC II and two-photon excitation spectra of lutein and β-carotene in solution: efficient Car S1→Chl electronic energy transfer via hot S1 states? J. Phys. Chem. A 106, 1909–1916 (2002).
Horton, P. et al. Control of the light-harvesting function of chloroplast membranes by aggregation of the LHCII chlorophyll–protein complex. FEBS Lett. 292, 1–4 (1991).
Ruban, A. V., Young, A. & Horton, P. Modulation of chlorophyll fluorescence quenching in isolated light harvesting complex of photosystem II. Biochim. Biophys. Acta Bioenerg. 1186, 123–127 (1994).
Ruban, A. V., Dekker, J. P., Horton, P. & van Grondelle, R. Temperature dependence of chlorophyll fluorescence from the light harvesting complex II of higher plants. Photochem. Photobiol. 61, 216–221 (1995).
Magdaong, N. M., Enriquez, M. M., LaFountain, A. M., Rafka, L. & Frank, H. A. Effect of protein aggregation on the spectroscopic properties and excited state kinetics of the LHCII pigment–protein complex from green plants. Photosynth. Res. 118, 259–276 (2013).
Ware, M. A., Giovagnetti, V., Belgio, E. & Ruban, A. V. PsbS protein modulates non-photochemical chlorophyll fluorescence quenching in membranes depleted of photosystems. J. Photochem. Photobiol. B 152, 301–307 (2015).
Krüger, T. P. J., Novoderezkhin, V. I., Ilioaia, C. & van Grondelle, R. Fluorescence spectral dynamics of single LHCII trimers. Biophys. J. 98, 3093–3101 (2010).
Lawton, W. H. & Sylvestre, E. A. Self modeling curve resolution. Technometrics 13, 617–633 (1971).
Chmeliov, J., Trinkunas, G., van Amerongen, H. & Valkunas, L. Light harvesting in a fluctuating antenna. J. Am. Chem. Soc. 136, 8963–8972 (2014).
Chmeliov, J., Trinkunas, G., van Amerongen, H. & Valkunas, L. Excitation migration in fluctuating light-harvesting antenna systems. Photosynth. Res. 127, 49–60 (2016).
Schatz, G. H., Brock, H. & Holzwarth, A. R. Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc. Natl Acad. Sci. USA 84, 8414–8418 (1987).
Dekker, J. P. & Boekema, E. J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta Bioenerg. 1706, 12–39 (2005).
Krüger, T. P. J., Ilioaia, C. & van Grondelle, R. Fluorescence intermittency from the main plant light-harvesting complex: resolving shifts between intensity levels. J. Phys. Chem. B 115, 5071–5082 (2011).
Krüger, T. P. J., Ilioaia, C., Valkunas, L. & van Grondelle, R. Fluorescence intermittency from the main plant light-harvesting complex: sensitivity to the local environment. J. Phys. Chem. B 115, 5083–5095 (2011).
Valkunas, L., Chmeliov, J., Krüger, T. P. J., Ilioaia, C. & van Grondelle, R. How photosynthetic proteins switch. J. Phys. Chem. Lett. 3, 2779–2784 (2012).
Chmeliov, J., Valkunas, L., Krüger, T. P. J., Ilioaia, C. & van Grondelle, R. Fluorescence blinking of single major light-harvesting complexes. New J. Phys. 15, 085007 (2013).
Novoderezhkin, V. I., Palacios, M. A., van Amerongen, H. & van Grondelle, R. Excitation dynamics in the LHCII complex of higher plants: modeling based on the 2.72 Å crystal structure. J. Phys. Chem. B 109, 10493–10504 (2005).
Müh, F., Madjet, M. E.-A. & Renger, T. Structure-based identification of energy sinks in plant light-harvesting complex II. J. Phys. Chem. B 114, 13517–13535 (2010).
Duffy, C. D. P. et al. Modeling of fluorescence quenching by lutein in the plant light-harvesting complex LHCII. J. Phys. Chem. B 117, 10974–10986 (2013).
Chmeliov, J. et al. An ‘all pigment’ model of excitation quenching in LHCII. Phys. Chem. Chem. Phys. 17, 15857–15867 (2015).
Barzda, V. et al. Singlet-singlet annihilation kinetics in aggregates and trimers of LHCII. Biophys. J. 80, 2409–2421 (2001).
Bennett, D. I. G., Amarnath, K. & Fleming, G. R. A structure-based model of energy transfer reveals the principles of light harvesting in photosystem II supercomplexes. J. Am. Chem. Soc. 135, 9164–9173 (2013).
Broess, K. et al. Excitation energy transfer and charge separation in photosystem II membranes revisited. Biophys. J. 91, 3776–3786 (2006).
Valkunas, L., Trinkunas, G., Chmeliov, J. & Ruban, A. V. Modeling of exciton quenching in photosystem II. Phys. Chem. Chem. Phys. 11, 7576–7584 (2009).
Valkunas, L. et al. Excitation migration, quenching, and regulation of photosynthetic light harvesting in photosystem II. J. Phys. Chem. B 115, 9252–9260 (2011).
Caffarri, S., Broess, K., Croce, R. & van Amerongen, H. Excitation energy transfer and trapping in higher plant photosystem II complexes with different antenna sizes. Biophys. J. 100, 2094–2103 (2011).
Mančal, T., Valkunas, L. & Fleming, G. R. Theory of exciton-charge transfer state coupled systems. Chem. Phys. Lett. 432, 301–305 (2006).
Wahadoszamen, M., Berera, R., Ara, A. M., Romero, E. & van Grondelle, R. Identification of two emitting sites in the dissipative state of the major light harvesting antenna. Phys. Chem. Chem. Phys. 14, 759–766 (2012).
Belgio, E., Johnson, M. P., Jurić, S. & Ruban, A. V. Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime—both the maximum and the nonphotochemically quenched. Biophys. J. 102, 2761–2771 (2012).
Moya, I., Silvestri, M., Vallon, O., Cinque, G. & Bassi, R. Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. Biochemistry 40, 12552–12561 (2001).
Ruban, A. V., Young, A. J., Pascal, A. A. & Horton, P. The effects of illumination on the xanthophyll composition of the photosystem II light-harvesting complexes of spinach thylakoid membranes. Plant Physiol. 104, 227–234 (1994).
Rutkauskas, D., Chmeliov, J., Johnson, M., Ruban, A. & Valkunas, L. Exciton annihilation as a probe of the light-harvesting antenna transition into the photoprotective mode. Chem. Phys. 404, 123–128 (2012).
Berry, M. W., Browne, M., Langville, A. N., Pauca, V. P. & Plemmons, R. J. Algorithms and applications for approximate nonnegative matrix factorization. Comput. Stat. Data Anal. 52, 155–173 (2007).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013); http://www.R-project.org/
Price, K. V., Storn, R. M. & Lampinen, J. A. Differential Evolution. A Practical Approach to Global Optimization (Natural Computing Series, Springer, 2005).
Ardia, D., Mullen, K. M., Peterson, B. G. & Ulrich, J. DEoptim: Differential Evolution in R. Version 2.2-3 (CRAN, 2015); http://CRAN.R-project.org/package=DEoptim
Acknowledgements
This work was supported by the Research Council of Lithuania (LMT grant no. MIP-080/2015). Computations were performed using the resources of the High Performance Computing Center ‘HPC Sauletekis’ at the Faculty of Physics, Vilnius University. A.V.R. would like to acknowledge grants from The Leverhulme Trust and UK Biotechnology and Biological Sciences Research Council and The Royal Society for the Wolfson Research Merit Award. The authors also thank E. Belgio and P. Ungerer for the LHCII purification.
Author information
Authors and Affiliations
Contributions
L.V. and A.V.R. designed the research. E.S. and R.A. performed the experiments. J.C. analysed experimental results in terms of self-modelling curve resolution, described high-temperature fluorescence kinetics in terms of fluctuating antenna model and made the figures of the manuscript. A.G. modelled excitation energy transfer in LHCII aggregates. L.V., R.A., J.C. and A.G. contributed to the interpretation of the experimental results. J.C. and A.G. wrote the manuscript. L.V., R.A., A.V.R. and C.D.P.D. discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Chmeliov, J., Gelzinis, A., Songaila, E. et al. The nature of self-regulation in photosynthetic light-harvesting antenna. Nature Plants 2, 16045 (2016). https://doi.org/10.1038/nplants.2016.45
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.45
This article is cited by
-
A perspective on the major light-harvesting complex dynamics under the effect of pH, salts, and the photoprotective PsbS protein
Photosynthesis Research (2023)
-
Towards the description of charge transfer states in solubilised LHCII using subsystem DFT
Photosynthesis Research (2023)
-
Recent progress in atomistic modeling of light-harvesting complexes: a mini review
Photosynthesis Research (2023)
-
Spruce versus Arabidopsis: different strategies of photosynthetic acclimation to light intensity change
Photosynthesis Research (2022)
-
The PsbS protein and low pH are necessary and sufficient to induce quenching in the light-harvesting complex of plants LHCII
Scientific Reports (2021)