Abstract
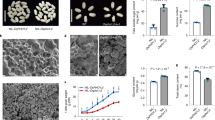
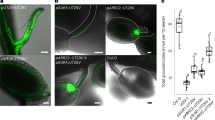
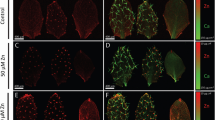
Insufficient intake of zinc and iron from a cereal-based diet is one of the causes of ‘hidden hunger’ (micronutrient deficiency), which affects some two billion people1,2. Identifying a limiting factor in the molecular mechanism of zinc loading into seeds is an important step towards determining the genetic basis for variation of grain micronutrient content and developing breeding strategies to improve this trait3. Nutrients are translocated to developing seeds at a rate that is regulated by transport processes in source leaves, in the phloem vascular pathway, and at seed sinks. Nutrients are released from a symplasmic maternal seed domain into the seed apoplasm surrounding the endosperm and embryo by poorly understood membrane transport processes4–6. Plants are unique among eukaryotes in having specific P1B-ATPase pumps for the cellular export of zinc7. In Arabidopsis, we show that two zinc transporting P1B-ATPases actively export zinc from the mother plant to the filial tissues. Mutant plants that lack both zinc pumps accumulate zinc in the seed coat and consequently have vastly reduced amounts of zinc inside the seed. Blockage of zinc transport was observed at both high and low external zinc supplies. The phenotype was determined by the mother plant and is thus due to a lack of zinc pump activity in the seed coat and not in the filial tissues. The finding that P1B-ATPases are one of the limiting factors controlling the amount of zinc inside a seed is an important step towards combating nutritional zinc deficiency worldwide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wessells, K. R. & Brown, K. H. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 7, e50568 (2012).
von Grebmer, K. et al. Global hunger index: the challenge of hidden hunger (International Food Policy Research Institute, 2014).
Palmgren, M. G. et al. Zinc biofortification of cereals problems and solutions. Trends Plant Sci. 13, 464–473 (2008).
Stadler, R., Lauterbach, C. & Sauer, N. Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol. 139, 701–712 (2005).
Zhang, W. et al. Nutrient loading of developing seeds. Funct. Plant Biol. 34, 314–331 (2007).
Radchuk, V. & Borisjuk, L. Physical, metabolic and developmental functions of the seed coat. Front. Plant Sci. 5, 510 (2014).
Williams, L. E. & Mills, R. F. P1B-ATPases—an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 10, 491–502 (2005).
Baxter, I. et al. Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 132, 618–628 (2003).
Kim, Y.-Y. et al. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant J. 58, 737–753 (2009).
Seigneurin-Berny, D. et al. HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions. J. Biol. Chem. 281, 2882–2892 (2006).
Morel, M. et al. AtHMA3, a P(1B)-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 149, 894–904 (2009).
Hussain, D. et al. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 16, 1327–1339 (2004).
Sinclair, S. A. et al. The use of the zinc-fluorophore, Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytol. 174, 39–45 (2007).
Verret, F. et al. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 576, 306–312 (2004).
Hanikenne, M. et al. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453, 391–396 (2008).
Kim, S. A. et al. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314, 1295–1298 (2006).
Schnell Ramos, M. et al. Using μPIXE for quantitative mapping of metal concentration in Arabidopsis thaliana seeds. Front Plant Sci. 4, 168 (2013).
Le, B. H. et al. Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl Acad. Sci. USA 107, 8063–8070 (2010).
Song, W.-Y. et al. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell 22, 2237–2252 (2010).
Morth, J. P. et al. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nature Rev. Mol. Cell Biol. 12, 60–70 (2011).
Mills, R. F. et al. HvHMA2, a P1B-ATPase from barley, is highly conserved among cereals and functions in Zn and Cd transport. PLoS One 7, e42640 (2012).
Tauris, B. et al. A roadmap for zinc trafficking in the developing barley grain based on laser capture microdissection and gene expression profiling. J. Exp. Bot. 60, 1333–1347 (2009).
Satoh-Nagasawa, N. et al. Mutations in rice (Oryza sativa) Heavy Metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 53, 213–224 (2012).
Takahashi, R. et al. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 35, 1948–1957 (2012).
Yamaji, N. et al. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 162, 927–939 (2013).
Barabasz, A. et al. Metal accumulation in tobacco expressing Arabidopsis halleri metal hyperaccumulation gene depends on external supply. J. Exp. Bot. 61, 3057–3067 (2010).
Siemianowski, O. et al. Expression of the P1B-type ATPase AtHMA4 in tobacco modifies Zn and Cd root to shoot partitioning and metal tolerance. Plant Biotechnol. J. 9, 64–74 (2011).
Cun, P. et al. Modulation of Zn/Cd P1B2-ATPase activities in Arabidopsis impacts differently on Zn and Cd contents in shoots and seeds. Metallomics 6, 2109–2916 (2014).
Kendziorek, M. et al. Approach to engineer tomato by expression of AtHMA4 to enhance Zn in the aerial parts. J. Plant Physiol. 171, 1413–1422 (2014).
Truernit, E. et al. High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 20, 1494–1503 (2008).
Acknowledgements
The authors thank C.S. Cobbett (University of Melbourne) for providing hma2-4, hma4-2, and hma2-4, hma4-2 mutant seeds and the Centre for Advanced Bioimaging (University of Copenhagen) for support and use of microscopes. We acknowledge the Paul Scherrer Institut, Villigen, Switzerland, for providing the synchrotron radiation beamtime at beamline microXAS of the SLS. The research leading to these results was funded by the University of Copenhagen's Excellency Programme KU2016, the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant agreement no. PIEF-GA-2012-331680, and the European programme CALIPSO (no. 312284).
Author information
Authors and Affiliations
Contributions
L.I.O., T.H.H., C.L., J.T.H., J.L., S.S., S.C. and V.S. performed the experimental work. L.I.O., T.H.H., C.L., R.D.H., J.L., S.S., U.K., S.H. and M.P. performed data analysis. D.G., U.K., S.H. and M.P. oversaw project planning. L.I.O. and M.P. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Olsen, L., Hansen, T., Larue, C. et al. Mother-plant-mediated pumping of zinc into the developing seed. Nature Plants 2, 16036 (2016). https://doi.org/10.1038/nplants.2016.36
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.36
This article is cited by
-
Cell type-specific mapping of ion distribution in Arabidopsis thaliana roots
Nature Communications (2023)
-
RETRACTED ARTICLE: Plant nutrient dynamics: a growing appreciation for the roles of micronutrients
Plant Growth Regulation (2023)
-
The evolution of plant proton pump regulation via the R domain may have facilitated plant terrestrialization
Communications Biology (2022)
-
Biofortification of Maize with Zinc and Its Effect on Human Health
Journal of Soil Science and Plant Nutrition (2022)
-
Arabidopsis bZIP19 and bZIP23 act as zinc sensors to control plant zinc status
Nature Plants (2021)