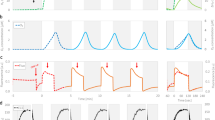
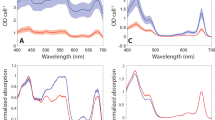
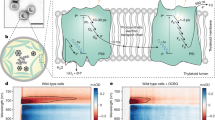
Abstract
Photosynthesis converts sunlight into biologically useful compounds, thus fuelling practically the entire biosphere. This process involves two photosystems acting in series powered by light harvesting complexes (LHCs) that dramatically increase the energy flux to the reaction centres. These complexes are the main targets of the regulatory processes that allow photosynthetic organisms to thrive across a broad range of light intensities. In microalgae, one mechanism for adjusting the flow of energy to the photosystems, state transitions, has a much larger amplitude than in terrestrial plants, whereas thermal dissipation of energy, the dominant regulatory mechanism in plants, only takes place after acclimation to high light. Here we show that, at variance with recent reports, microalgal state transitions do not dissipate light energy but redistribute it between the two photosystems, thereby allowing a well-balanced influx of excitation energy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Archer, M. D. & Barber, J. Molecular to Global Photosynthesis (Imperial College Press, 2004).
Croce, R. & van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nature Chem. Biol. 10, 492–501 (2014).
Rochaix, J.-D. Regulation and dynamics of the light-harvesting system. Annu. Rev. Plant Biol. 65, 287–309 (2014).
Goss, R. & Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 172, 13–32 (2015).
Nawrocki, W. J., Tourasse, N. J., Taly, A., Rappaport, F. & Wollman, F. A. The plastid terminal oxidase: its elusive function points to multiple contributions to plastid physiology. Ann. Rev. Plant Biol. 66, 49–74 (2015).
Bonaventura, C. & Myers, J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim. Biophys. Acta 189, 366–383 (1969).
Murata, N. Control of excitation transfer in photosynthesis: Light-induced change of chlorophyll a fluorescence in porphyridium cruentum. Biochim. Biophys. Acta 172, 242–251 (1969).
Wollman, F. A. State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 20, 3623–3630 (2001).
Goldschmidt-Clermont, M. & Bassi, R. Sharing light between two photosystems: mechanism of state transitions. Curr. Opin. Plant Biol. 25, 71–78 (2015).
Kyle, D. J., Staehelin, L. A. & Arntzen, C. J. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation-energy distribution in higher-plants. Arch. Biochem. Biophys. 222, 527–541 (1983).
Vallon, O., Wollman, F. A. & Olive, J. Lateral distribution of the main protein complexes of the photosynthetic apparatus in Chlamydomonas-reinhardtii and in spinach—an immunocytochemical study using intact thylakoid membranes and a PS-II enriched membrane preparation. Photobiochem. Photobiophys. 12, 203–220 (1986).
Cardol, P. et al. Impaired respiration discloses the physiological significance of state transitions in Chlamydomonas. Proc. Natl Acad. Sci. USA 106, 15979–15984 (2009).
Allen, J. F. Protein-phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098, 275–335 (1992).
Iwai, M., Yokono, M., Inada, N. & Minagawa, J. Live-cell imaging of photosystem II antenna dissociation during state transitions. Proc. Natl Acad. Sci. USA 107, 2337–2342 (2010).
Unlu, C., Drop, B., Croce, R. & van Amerongen, H. state transitions in Chlamydomonas reinhardtii strongly modulate the functional size of photosystem II but not of photosystem I. Proc. Natl Acad. Sci. USA 111, 3460–3465 (2014).
Nagy, G. et al. Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo. Proc. Natl Acad. Sci. USA 111, 5042–5047 (2014).
Unlu, C., Polukhina, I. & van Amerongen, H. Origin of pronounced differences in 77 K fluorescence of the green alga Chlamydomonas reinhardtii in state 1 and 2. Eur. Biophys. J. http://dx.doi.org/10.1007/s00249-015-1087-9 (2015).
The Chlamydomonas Sourcebook 2nd edn (eds Harris, E. H., Stern, D. B. & Witman, G. B. ) 1–24 (Academic Press, 2009).
Peers, G. et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462, 518–U215 (2009). .
Delosme, R., Olive, J. & Wollman, F. A. Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta-Bioenerg. 1273, 150–158 (1996).
Houille-Vernes, L., Rappaport, F., Wollman, F. A., Alric, J. & Johnson, X. Plastid terminal oxidase 2 (PTOX2) is the major oxidase involved in chlororespiration in Chlamydomonas. Proc. Natl Acad. Sci. USA 108, 20820–20825 (2011).
Delepelaire, P. & Wollman, F. A. Correlations between fluorescence and phosphorylation changes in thylakoid membranes of Chlamydomonas-reinhardtii in vivo—a kinetic-analysis. Biochim. Biophys. Acta 809, 277–283 (1985).
Witt, H. T. Energy-conversion in the functional membrane of photosynthesis – analysis by light-pulse and electric pulse methods - central role of the electric-field. Biochim. Biophys. Acta 505, 355–427 (1979).
Murakami, A. Quantitative analysis of 77K fluorescence emission spectra in Synechocystis sp. PCC 6714 and Chlamydomonas reinhardtii with variable PS I/PS II stoichiometries. Photosynth. Res. 53, 141–148 (1997).
Lunde, C., Jensen, P. E., Haldrup, A., Knoetzel, J. & Scheller, H. V. The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408, 613–615 (2000).
Rappaport, F., Beal, D., Joliot, A. & Joliot, P. On the advantages of using green light to study fluorescence yield changes in leaves. Biochim. Biophys. Acta 1767, 56–65 (2007).
Rizzo, F., Zucchelli, G., Jennings, R. & Santabarbara, S. Wavelength dependence of the fluorescence emission under conditions of open and closed Photosystem II reaction centres in the green alga Chlorella sorokiniana. Biochim. Biophys. Acta-Bioenerg. 1837, 726–733 (2014).
Drop, B., Yadav, K. N. S., Boekema, E. J. & Croce, R. Consequences of state transitions on the structural and functional organization of photosystem I in the green alga Chlamydomonas reinhardtii. Plant J. 78, 181–191 (2014).
Takahashi, H., Okamuro, A., Minagawa, J. & Takahashi, Y. Biochemical characterization of photosystem I-associated light-harvesting complexes I and II isolated from state 2 cells of Chlamydomonas reinhardtii. Plant Cell Physiol. 55, 1437–1449 (2014).
Galka, P. et al. Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 24, 2963–2978 (2012).
Le Quiniou, C. et al. PSI-LHCI of Chlamydomonas reinhardtii: Increasing the absorption cross section without losing efficiency. Biochim. Biophys. Acta 1847, 458–467 (2015).
Wlodarczyk, L. M. et al. Functional rearrangement of the light-harvesting antenna upon state transitions in a green alga. Biophys. J. 108, 261–271 (2015).
Takahashi, H., Iwai, M., Takahashi, Y. & Minagawa, J. Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA 103, 477–482 (2006).
Johnson, X. & Alric, J. Interaction between starch breakdown, acetate assimilation, and photosynthetic cyclic electron flow in Chlamydomonas reinhardtii. J. Biol. Chem. 287, 26445–26452 (2012).
Clowez, S., Godaux, D., Cardol, P., Wollman, F. A. & Rappaport, F. The involvement of hydrogen-producing and ATP-dependent NADPH-consuming pathways in setting the redox poise in the chloroplast of Chlamydomonas reinhardtii in anoxia. J. Biol. Chem. 290, 8666–8676 (2015).
Godaux, D., Bailleul, B., Berne, N. & Cardol, P. Induction of photosynthetic carbon fixation in anoxia relies on hydrogenase activity and proton-gradient regulation-like1-mediated cyclic electron flow in Chlamydomonas reinhardtii. Plant Physiol. 168, 648–658 (2015).
Quinn, J. et al. The plastocyanin-deficient phenotype of Chlamydomonas reinhardtii Ac-208 results from a frame-shift mutation in the nuclear gene encoding preapoplastocyanin. J. Biol. Chem. 268, 7832–7841 (1993).
Gallaher, S. D., Fitz-Gibbon, S. T., Glaesener, A. G., Pellegrini, M. & Merchant, S. S. Chlamydomonas genome resource for laboratory strains reveals a mosaic of sequence variation, identifies true strain histories, and enables strain-specific studies. Plant Cell 27, 2335–2352 (2015).
Redding, K. et al. A systematic survey of conserved histidines in the core subunits of Photosystem I by site-directed mutagenesis reveals the likely axial ligands of P-700. EMBO J. 17, 50–60 (1998).
Joliot, P. & Joliot, A. Quantification of cyclic and linear flows in plants. Proc. Natl Acad. Sci. USA 102, 4913–4918 (2005).
Alric, J., Lavergne, J. & Rappaport, F. Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii (I) aerobic conditions. Biochim. Biophys. Acta-Bioenerg. 1797, 44–51 (2010).
Takahashi, H., Clowez, S., Wollman, F. A., Vallon, O. & Rappaport, F. Cyclic electron flow is redox-controlled but independent of state transition. Nature Commun. 4, 1954 (2013).
Engel, B. D. et al. Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. eLife 4 (2015).
Drop, B. et al. Light-harvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1837, 63–72 (2014).
Drop, B. et al. Photosystem I of Chlamydomonas reinhardtii contains nine light-harvesting complexes (Lhca) located on one side of the core. J. Biol. Chem. 286, 44878–44887 (2011).
Barber, J. Regulation of energy-transfer by cations and protein-phosphorylation in relation to thylakoid membrane organization. Photosynth. Res. 10, 243–253 (1986).
Kargul, J. et al. Light-harvesting complex II protein CP29 binds to photosystem I of Chlamydomonas reinhardtii under state 2 conditions. FEBS J. 272, 4797–4806 (2005).
Suga, M. et al. Native structure of photosystem II at 1.95 A resolution viewed by femtosecond X-ray pulses. Nature 517, 99–103 (2015).
Mazor, Y., Borovikova, A. & Nelson, N. The structure of plant photosystem I super-complex at 2.8 A resolution. eLife 4, e07433 (2015).
Tokutsu, R., Kato, N., Bui, K. H., Ishikawa, T. & Minagawa, J. Revisiting the supramolecular organization of photosystem II in Chlamydomonas reinhardtii. J. Biol. Chem. 287, 31574–31581 (2012).
Acknowledgements
We thank S. Bujaldon for the ΔPC-WT cross. W.J.N., F.-A.W. and F.R. acknowledge the support of CNRS and Université Pierre et Marie Curie. This work was supported by the Initiative d'Excellence programme from the French State (grant DYNAMO, ANR-11-LABX-0011-01) and by the programme PHC PROCOPE 2014 PROJET no. 30729YB. W.J.N. is a recipient of a PhD fellowship from the Université Pierre et Marie Curie. S.S. acknowledges the support from the DYNAMO ANR-11-LABX-0011-01 grant for travel. L.M. was a recipient of the Studienstiftung des Deutschen Volkes fellowship for her Master studies. Fabrice Rappaport deceased before final acceptance of this manuscript. His co-authors wish to acknowledge his remarkable contributions to our current understanding of photosynthesis regulations.
Author information
Authors and Affiliations
Contributions
W.J.N., S.S., L.M. performed the experiments. F.-A.W. and F.R. conceived and designed the research. All of the authors discussed, analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Nawrocki, W., Santabarbara, S., Mosebach, L. et al. State transitions redistribute rather than dissipate energy between the two photosystems in Chlamydomonas. Nature Plants 2, 16031 (2016). https://doi.org/10.1038/nplants.2016.31
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.31
This article is cited by
-
Weak acids produced during anaerobic respiration suppress both photosynthesis and aerobic respiration
Nature Communications (2023)
-
Reversible protein phosphorylation in higher plants: focus on state transitions
Biophysical Reviews (2023)
-
Structural basis of LhcbM5-mediated state transitions in green algae
Nature Plants (2021)
-
Ultrafast excited-state dynamics in land plants Photosystem I core and whole supercomplex under oxidised electron donor conditions
Photosynthesis Research (2020)
-
Structural insight into light harvesting for photosystem II in green algae
Nature Plants (2019)