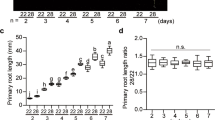
Abstract
A hallmark of plants is their adaptability of size and form in response to widely fluctuating environments. The metabolism and redistribution of the phytohormone auxin play pivotal roles in establishing active auxin gradients and resulting cellular differentiation. In Arabidopsis thaliana, cotyledons and leaves synthesize indole-3-acetic acid (IAA) from tryptophan through indole-3-pyruvic acid (3-IPA) in response to vegetational shade. This newly synthesized auxin moves to the hypocotyl where it induces elongation of hypocotyl cells. Here we show that loss of function of VAS2 (IAA-amido synthetase Gretchen Hagen 3 (GH3).17) leads to increases in free IAA at the expense of IAA-Glu (IAA-glutamate) in the hypocotyl epidermis. This active IAA elicits shade- and high temperature-induced hypocotyl elongation largely independently of 3-IPA-mediated IAA biosynthesis in cotyledons. Our results reveal an unexpected capacity of local auxin metabolism to modulate the homeostasis and spatial distribution of free auxin in specialized organs such as hypocotyls in response to shade and high temperature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Franklin, K. A. Shade avoidance. New Phytol. 179, 930–944 (2008).
Casal, J. J. Shade avoidance. Arabidopsis Book 10, e0157 (2012).
Li, L. et al. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 26, 785–790 (2012).
Tao, Y. et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176 (2008).
de Wit, M., Lorrain, S. & Fankhauser, C. Auxin-mediated plant architectural changes in response to shade and high temperature. Physiol. Plant 151, 13–24 (2014).
Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008).
Sun, J., Qi, L., Li, Y., Chu, J. & Li, C. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet. 8, e1002594 (2012).
Keuskamp, D. H., Pollmann, S., Voesenek, L. A., Peeters, A. J. & Pierik, R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl Acad. Sci. USA 107, 22740–22744 (2010).
Franklin, K. A. et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl Acad. Sci. USA 108, 20231–20235 (2011).
Yamada, M., Greenham, K., Prigge, M. J., Jensen, P. J. & Estelle, M. The TRANSPORT INHIBITOR RESPONSE2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiol. 151, 168–179 (2009).
Procko, C., Crenshaw, C. M., Ljung, K., Noel, J. P. & Chory, J. Cotyledon-generated auxin is required for shade-induced hypocotyl growth in Brassica rapa. Plant Physiol. 165, 1285–1301 (2014).
Petrasek, J. & Friml, J. Auxin transport routes in plant development. Development 136, 2675–2688 (2009).
Zheng, Z. et al. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nature Chem. Biol. 9, 244–246 (2013).
Hagen, G., Kleinschmidt, A. & Guilfoyle, T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 162, 147–153 (1984).
Staswick, P. E. et al. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17, 616–627 (2005).
Westfall, C. S., Herrmann, J., Chen, Q., Wang, S. & Jez, J. M. Modulating plant hormones by enzyme action: the GH3 family of acyl acid amido synthetases. Plant Signal. Behav. 5, 1607–1612 (2010).
Ludwig-Muller, J. Auxin conjugates: their role for plant development and in the evolution of land plants. J. Exp. Bot. 62, 1757–1773 (2011).
Korasick, D. A., Enders, T. A. & Strader, L. C. Auxin biosynthesis and storage forms. J. Exp. Bot. 64, 2541–2555 (2013).
Morant, M. et al. Metabolomic, transcriptional, hormonal, and signaling cross-talk in superroot2. Mol. Plant 3, 192–211 (2010).
Paponov, I. A. et al. Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol. Plant 1, 321–337 (2008).
Terol, J., Domingo, C. & Talon, M. The GH3 family in plants: genome wide analysis in rice and evolutionary history based on EST analysis. Gene 371, 279–290 (2006).
Novak, O. et al. Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. Plant J. 72, 523–536 (2012).
Ljung, K. Auxin metabolism and homeostasis during plant development. Development 140, 943–950 (2013).
Woodward, A. W. & Bartel, B. Auxin: regulation, action, and interaction. Ann. Bot. 95, 707–735 (2005).
Rampey, R. A. et al. A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiol. 135, 978–988 (2004).
LeClere, S., Tellez, R., Rampey, R. A., Matsuda, S. P. & Bartel, B. Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J. Biol. Chem. 277, 20446–20452 (2002).
Sauer, M., Robert, S. & Kleine-Vehn, J . Auxin: simply complicated. J. Exp. Bot. 64, 2565–2577 (2013).
Kim, J. Y. et al. Identification of an ABCB/P-glycoprotein-specific inhibitor of auxin transport by chemical genomics. J. Biol. Chem. 285, 23309–23317 (2010).
Pencik, A. et al. Regulation of auxin homeostasis and gradients in Arabidopsis roots through the formation of the indole-3-acetic acid catabolite 2-oxindole-3-acetic acid. Plant Cell 25, 3858–3870 (2013).
Khan, S. & Stone, J. M. Arabidopsis thaliana GH3.9 influences primary root growth. Planta 226, 21–34 (2007).
Westfall, C. S. et al. Structural basis for prereceptor modulation of plant hormones by GH3 proteins. Science 336, 1708–1711 (2012).
Yuan, H. et al. Genome-wide analysis of the GH3 family in apple (Malus × domestica). BMC Genomics 14, 297 (2013).
Fu, J., Yu, H., Li, X., Xiao, J. & Wang, S. Rice GH3 gene family: regulators of growth and development. Plant Signal. Behav. 6, 570–574 (2011).
Khan, S. & Stone, J. M. Arabidopsis thaliana GH3.9 in auxin and jasmonate cross talk. Plant Signal. Behav. 2, 483–485 (2007).
Mittag, J., Gabrielyan, A. & Ludwig-Muller, J. Knockout of GH3 genes in the moss Physcomitrella patens leads to increased IAA levels at elevated temperature and in darkness. Plant Physiol. Biochem. 97, 339–349 (2015).
Jagadeeswaran, G. et al. Arabidopsis GH3-LIKE DEFENSE GENE 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae. Plant J. 51, 234–246 (2007).
Takase, T., Nakazawa, M., Ishikawa, A., Manabe, K. & Matsui, M. DFL2, a new member of the Arabidopsis GH3 gene family, is involved in red light-specific hypocotyl elongation. Plant Cell Physiol. 44, 1071–1080 (2003).
Nakazawa, M. et al. DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 25, 213–221 (2001).
Santner, A., Calderon-Villalobos, L. I. & Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nature Chem. Biol. 5, 301–307 (2009).
Nemhauser, J. L., Hong, F. & Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126, 467–475 (2006).
Zhao, Y. Auxin biosynthesis. Arabidopsis Book 12, e0173 (2014).
van Berkel, K., de Boer, R. J., Scheres, B. & ten Tusscher, K. Polar auxin transport: models and mechanisms. Development 140, 2253–2268 (2013).
Blilou, I. et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44 (2005).
Grieneisen, V. A., Xu, J., Maree, A. F., Hogeweg, P. & Scheres, B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449, 1008–1013 (2007).
Pinon, V., Prasad, K., Grigg, S. P., Sanchez-Perez, G. F. & Scheres, B. Local auxin biosynthesis regulation by PLETHORA transcription factors controls phyllotaxis in Arabidopsis. Proc. Natl Acad. Sci. USA 110, 1107–1112 (2013).
Mravec, J. et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459, 1136–1140 (2009).
Barbez, E. et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 485, 119–122 (2012).
Morgan, D. C., O'Brien, T. & Smith, H. Rapid photomodulation of stem extension in light-grown Sinapis alba L.: studies on kinetics, site of perception and photoreceptor. Planta 150, 95–101 (1980).
Preuten, T., Hohm, T., Bergmann, S. & Fankhauser, C. Defining the site of light perception and initiation of phototropism in Arabidopsis. Curr. Biol. 23, 1934–1938 (2013).
Yamamoto, Y. et al. Quality control of PSII: behavior of PSII in the highly crowded grana thylakoids under excessive light. Plant Cell Physiol. 55, 1206–1215 (2014).
Acknowledgements
We thank M. Geisler for the generous gift of BUM, and the Arabidopsis Biological Resource Center for mutant seeds. These studies were supported by US National Institutes of Health (NIH) grant 5R01GM52413 to J.C., the Swedish Governmental Agency for Innovation Systems (VINNOVA) and the Swedish Research Council (VR) to K.L. and O.N., the Ministry of Education, Youth and Sports of the Czech Republic (the National Program for Sustainability I No. LO1204) to O.N., the National Science Foundation under award nos EEC-0813570 and MCB-0645794 to J.P.N. and the Howard Hughes Medical Institute (Z.Z., J.P.N., J.C.).
Author information
Authors and Affiliations
Contributions
Z.Y.Z. designed the experiments with the input from J.P.N. and J.C., Z.Y.Z., Y.X.G., O.N., W.C. and K.L. performed the experiments. Z.Y.Z., J.P.N. and J.C. wrote the manuscript. All authors discussed the results and commented on the experimental design.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, Z., Guo, Y., Novák, O. et al. Local auxin metabolism regulates environment-induced hypocotyl elongation. Nature Plants 2, 16025 (2016). https://doi.org/10.1038/nplants.2016.25
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.25
This article is cited by
-
Genome-wide association study for temperature response and photo-thermal interaction of flowering time in soybean using a panel of cultivars with diverse maturity groups
Theoretical and Applied Genetics (2023)
-
Molecular breeding for improvement of photothermal adaptability in soybean
Molecular Breeding (2023)
-
PIF7 is a master regulator of thermomorphogenesis in shade
Nature Communications (2022)
-
A combination of plasma membrane sterol biosynthesis and autophagy is required for shade-induced hypocotyl elongation
Nature Communications (2022)
-
Role of Phytohormones in Regulating Heat Stress Acclimation in Agricultural Crops
Journal of Plant Growth Regulation (2022)