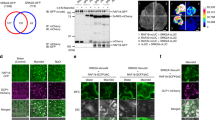
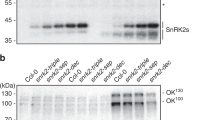
Abstract
Rapid changes in messenger RNA population are vital for plants to properly exert multiple adaptive responses under continuously changing stress conditions. Transcriptional activation mediated by the ‘abscisic acid (ABA)-activated SnRK2 protein kinases–ABA-responsive element (ABRE)-binding proteins/ABRE-binding factors (AREB/ABFs)’ signalling module is a crucial step in the expression of stress-inducible genes under osmotic stress conditions in Arabidopsis1–4. In addition to transcriptional control, proper transcript levels of individual genes can be achieved by post-transcriptional regulation, but how this regulation functions under stress conditions and the underlying molecular mechanisms remain elusive. Here, we show that ABA-unresponsive osmotic stress-activated subclass I SnRK2s and their downstream substrate, VARICOSE (VCS), an mRNA decapping activator, regulate mRNA decay under osmotic stress conditions. The expression of many stress-responsive genes was similarly misregulated in a mutant lacking all functional subclass I SnRK2s and in VCS-knockdown plants. Additionally, the mRNA decay of the transcripts of these genes was impaired in these plants under osmotic stress conditions. Furthermore, these plants showed growth retardation under osmotic stresses. Notably, subclass I-type SnRK2s have been identified in seed plants but not in lycophytes or mosses. Therefore, the post-transcriptional regulation mediated by the ‘subclass I SnRK2s–VARICOSE’ signalling module represents an additional mechanism of gene expression control that facilitates drastic changes in mRNA populations under osmotic stresses and might enhance the adaptability of seed plants to stress conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Furihata, T. et al. Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc. Natl Acad. Sci. USA 103, 1988–1993 (2006).
Fujii, H. & Zhu, J. K. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl Acad. Sci. USA 106, 8380–8385 (2009).
Fujita, Y. et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 50, 2123–2132 (2009).
Fujii, H. et al. In vitro reconstitution of an abscisic acid signalling pathway. Nature 462, 660–664 (2009).
Park, S. Y. et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071 (2009).
Ma, Y. et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324, 1064–1068 (2009).
Nishimura, N. et al. Structural mechanism of abscisic acid binding and signaling by dimeric PYR1. Science 326, 1373–1379 (2009).
Santiago, J. et al. The abscisic acid receptor PYR1 in complex with abscisic acid. Nature 462, 665–668 (2009).
Miyazono, K. et al. Structural basis of abscisic acid signalling. Nature 462, 609–614 (2009).
Umezawa, T. et al. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl Acad. Sci. USA 106, 17588–17593 (2009).
Yoshida, T. et al. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 38, 35–49 (2015).
Yoshida, T., Mogami, J. & Yamaguchi-Shinozaki, K. Omics approaches toward defining the comprehensive abscisic acid signaling network in plants. Plant Cell Physiol. 56, 1043–1052 (2015).
Boudsocq, M., Barbier-Brygoo, H. & Laurière, C. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J. Biol. Chem. 279, 41758–41766 (2004).
Fujita, Y., Yoshida, T. & Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 147, 15–27 (2013).
Fujii, H., Verslues, P. E. & Zhu, J. K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc. Natl Acad. Sci. USA 108, 1717–1722 (2011).
McLoughlin, F. et al. The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J. 72, 436–449 (2012).
Xu, J., Yang, J. Y., Niu, Q. W. & Chua, N. H. Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell 18, 3386–3398 (2006).
Umezawa, T. et al. Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci. Signal 6, rs8 (2013).
Wang, P. et al. Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc. Natl Acad. Sci. USA 110, 11205–11210 (2013).
Stecker, K. E., Minkoff, B. B. & Sussman, M. R. Phosphoproteomic analyses reveal early signaling events in the osmotic stress response. Plant Physiol. 165, 1171–1187 (2014).
Deyholos, M. K. et al. VARICOSE, a WD-domain protein, is required for leaf blade development. Development 130, 6577–6588 (2003).
Goeres, D. C. et al. Components of the Arabidopsis mRNA decapping complex are required for early seedling development. Plant Cell 19, 1549–1564 (2007).
Xu, J. & Chua, N. H. Arabidopsis decapping 5 is required for mRNA decapping, P-body formation, and translational repression during postembryonic development. Plant Cell 21, 3270–3279 (2009).
Clough, S. J. Floral dip: agrobacterium-mediated germ line transformation. Methods Mol. Biol. 286, 91–102 (2005).
Waadt, R. et al. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 56, 505–516 (2008).
Mogami, J. et al. Two distinct families of protein kinases are required for plant growth under high external Mg2+ concentrations in Arabidopsis. Plant Physiol. 167, 1039–1057 (2015).
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J. & Zhu, J. K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45, 523–539 (2006).
Roux, M. E. et al. The mRNA decay factor PAT1 functions in a pathway including MAP kinase 4 and immune receptor SUMM2. EMBO J. 34, 593–608 (2015).
Acknowledgements
The authors thank Y. Tanaka, A. Watanabe and S. Mizukado for excellent technical assistance, E. Toma for skilful editorial assistance, T. Umezawa and M. Mizoguchi for providing the srk2abgh mutant line and Y. Fujita for providing the pGreenII0229-NosT vector. This work was financially supported by grants from a Grant-in-Aid for Scientific Research on Innovative Areas (no. JP15H05960 to K. Y.-S.) and for Scientific Research (A) (no. JP25251031 to K. Y.-S.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Program for the Promotion of Basic Research Activities for Innovative Biosciences (BRAIN) of Japan (to K. S. and K. Y.-S.).
Author information
Authors and Affiliations
Contributions
F.S., J.Mo. and K.Y.-S. designed the research. F.S. and J.Mo. contributed equally. F.S., J.Mo., T.Y., M.A., F.T. and S.K. performed the experiments. F.S., J.Mo., T.Y., M.A., F.T. and J.Mi. analysed the data. F.S., J.Mo., K.S. and K.Y.-S. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–12. (PDF 56735 kb)
Supplementary Table 1
Candidate SRK2G-interacting proteins were identified in samples from untreated plants using LC-MS/MS analyses. (XLSX 204 kb)
Supplementary Table 2
Many candidate SRK2G-interacting proteins were detected in extracts from SRK2G-GFP plants treated with 0.8 M mannitol. (XLSX 12 kb)
Supplementary Table 3
List of genes showing increased expression levels in the srk2abgh mutant in comparison to the expression levels in wild-type plants after treatment with dehydration for 5 h. (XLSX 49 kb)
Supplementary Table 4
Comparison of mRNA stability of subclass I SnRK2-regulated genes within each genotype under control and high-salinity conditions. (XLSX 53 kb)
Supplementary Table 5
Primer pairs used in this study. (XLSX 40 kb)
Rights and permissions
About this article
Cite this article
Soma, F., Mogami, J., Yoshida, T. et al. ABA-unresponsive SnRK2 protein kinases regulate mRNA decay under osmotic stress in plants. Nature Plants 3, 16204 (2017). https://doi.org/10.1038/nplants.2016.204
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.204
This article is cited by
-
Deciphering the regulatory role of PheSnRK genes in Moso bamboo: insights into hormonal, energy, and stress responses
BMC Genomics (2024)
-
GmWRKY17-mediated transcriptional regulation of GmDREB1D and GmABA2 controls drought tolerance in soybean
Plant Molecular Biology (2023)
-
Genome-wide identification and comparative analysis of the PYL gene family in eight Rosaceae species and expression analysis of seeds germination in pear
BMC Genomics (2022)
-
Plant hormone regulation of abiotic stress responses
Nature Reviews Molecular Cell Biology (2022)
-
Genome-wide identification and expression analysis of sucrose nonfermenting-1-related protein kinase (SnRK) genes in Triticum aestivum in response to abiotic stress
Scientific Reports (2021)