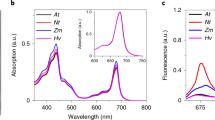
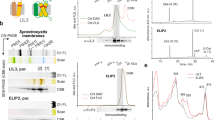
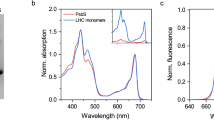
Abstract
Light-harvesting complexes (LHCs) are major constituents of the antenna systems in higher plant photosystems. Four Lhca subunits are tightly bound to the photosystem I (PSI) core complex, forming its outer antenna moiety called LHCI. The Arabidopsis thaliana mutant ΔLhca lacks all Lhca1–4 subunits and compensates for its decreased antenna size by binding LHCII trimers, the main constituent of the photosystem II antenna system, to PSI. In this work we have investigated the effect of LHCI/LHCII substitution by comparing the light harvesting and excitation energy transfer efficiency properties of PSI complexes isolated from ΔLhca mutants and from the wild type, as well as the consequences for plant growth. We show that the excitation energy transfer efficiency was not compromised by the substitution of LHCI with LHCII but a significant reduction in the absorption cross-section was observed. The absence of LHCI subunits in PSI thus significantly limits light harvesting, even on LHCII binding, inducing, as a consequence, a strong reduction in growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Qin, X., Suga, M., Kuang, T. & Shen, J. R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348, 989–995 (2015).
Mazor, Y., Borovikova, A. & Nelson, N. The structure of plant photosystem I super-complex at 2.8 Å resolution. Elife 4, e07433 (2015).
Croce, R. & van Amerongen, H. Light-harvesting in photosystem I. Photosynth. Res. 116, 153–166 (2013).
van Amerongen, H. & Croce, R. Light harvesting in photosystem II. Photosynth. Res. 116, 251–263 (2013).
Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4, 236–240 (1999).
Ballottari, M., Girardon, J., Dall'Osto, L. & Bassi, R. Evolution and functional properties of Photosystem II light harvesting complexes in eukaryotes. Biochim. Biophys. Acta 1817, 143–157 (2012).
Ballottari, M., Govoni, C., Caffarri, S. & Morosinotto, T. Stoichiometry of LHCI antenna polypeptides and characterization of gap and linker pigments in higher plants photosystem I. Eur. J. Biochem. 271, 4659–4665 (2004).
Ben-Shem, A., Frolow, F. & Nelson, N. Crystal structure of plant photosystem I. Nature 426, 630–635 (2003).
Amunts, A., Toporik, H., Borovikova, A. & Nelson, N. Structure determination and improved model of plant photosystem I. J. Biol. Chem. 285, 3478–3486 (2010).
Ballottari, M., Dall'Osto, L., Morosinotto, T. & Bassi, R. Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J. Biol. Chem. 282, 8947–8958 (2007).
Williams, W. P. & Salamon, Z. Enhancement studies on algae and isolated chloroplasts. Part I.: Variability of photosynthetic enhancement in Chlorella pyrenoidosa. Biochim. Biophys. Acta. 430, 282–299 (1976).
Hodges, M. & Barber, J. State 1-state 2 transitions in a unicellular green algae: analysis of in vivo chlorophyll fluorescence induction curves in the presence of 3-(3,4-dichlorophenyl)-1, 1-dimethylurea (DCMU). Plant Physiol. 72, 1119–1122 (1983).
Wollman, F. A. State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 20, 3623–3630 (2001).
Allen, J. F. Botany. State transitions--a question of balance. Science 299, 1530–1532 (2003).
Grieco, M., Suorsa, M., Jajoo, A., Tikkanen, M. & Aro, E. M. Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery – including both photosystems II and I. Biochim. Biophys. Acta 1847, 607–619 (2015).
Goldschmidt-Clermont, M. & Bassi, R. Sharing light between two photosystems: mechanism of state transitions. Curr. Opin. Plant Biol. 25, 71–78 (2015).
Wientjes, E., Drop, B., Kouřil, R., Boekema, E. J. & Croce, R. During state 1 to state 2 transition in Arabidopsis thaliana, the photosystem II supercomplex gets phosphorylated but does not disassemble. J. Biol. Chem. 288, 32821–6 (2013).
Bellafiore, S., Barneche, F., Peltier, G. & Rochaix, J. D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895 (2005).
Depège, N., Bellafiore, S. & Rochaix, J. D. Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299, 1572–1575 (2003).
Pesaresi, P. et al. Arabidopsis STN7 kinase provides a link between short- and long-term photosynthetic acclimation. Plant Cell 21, 2402–2423 (2009).
Pesaresi, P. et al. Optimizing photosynthesis under fluctuating light: the role of the Arabidopsis STN7 kinase. Plant Signal. Behav. 5, 21–25 (2010).
Rochaix, J. D. et al. Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment. Philos. Trans. R. Soc. Lond. B 367, 3466–3474 (2012).
Pribil, M., Pesaresi, P., Hertle, A., Barbato, R. & Leister, D. Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol. 8, e1000288 (2010).
Shapiguzov, A. et al. The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl Acad. Sci. USA 107, 4782–4787 (2010).
Wientjes, E., van Amerongen, H. & Croce, R. LHCII is an antenna of both photosystems after long-term acclimation. Biochim. Biophys. Acta 1827, 420–426 (2013).
Galka, P. et al. Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 24, 2963–2978 (2012).
Akhtar, P. et al. Excitation energy transfer between light-harvesting complex II and photosystem I in reconstituted membranes. Biochim. Biophys. Acta 1857, 462–472 (2016).
Wientjes, E., Oostergetel, G. T., Jansson, S., Boekema, E. J. & Croce, R. The role of Lhca complexes in the supramolecular organization of higher plant photosystem I. J. Biol. Chem. 284, 7803–7810 (2009).
Morosinotto, T., Ballottari, M., Klimmek, F., Jansson, S. & Bassi, R. The association of the antenna system to photosystem I in higher plants. Cooperative interactions stabilize the supramolecular complex and enhance red-shifted spectral forms. J. Biol. Chem. 280, 31050–31058 (2005).
Malkin, S., Armond, P. A., Mooney, H. A. & Fork, D. C. Photosystem II photosynthetic unit sizes from fluorescence induction in leaves: correlation to photosynthetic capacity. Plant Physiol. 67, 570–579 (1981).
Järvi, S., Suorsa, M., Paakkarinen, V. & Aro, E. M. Optimized native gel systems for separation of thylakoid protein complexes: novel super- and mega-complexes. Biochem. J. 439, 207–214 (2011).
Allen, J. F. Plastoquinone redox control of chloroplast thylakoid protein phosphorylation and distribution of excitation energy between photosystems: discovery, background, implications. Photosynth. Res. 73, 139–148 (2002).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 428, 287–292 (2004).
Standfuss, J., Terwisscha van Scheltinga, A. C., Lamborghini, M. & Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 24, 919–928 (2005).
Bassi, R., Croce, R., Cugini, D. & Sandonà, D. Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl Acad. Sci. USA 96, 10056–10061 (1999).
Kouril, R. et al. Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 44, 10935–10940 (2005).
Morosinotto, T., Breton, J., Bassi, R. & Croce, R. The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I. J. Biol. Chem. 278, 49223–49229 (2003).
Rivadossi, A., Zucchelli, G., Garlaschi, F. M. & Jennings, R. C. The importance of PSI chlorophyll red forms in light-harvesting by leaves. Photosynt. Res. 60, 209–215 (1999).
Laisk, A., Oja, V., Eichelmann, H. & Dall'Osto, L. Action spectra of photosystems II and I and quantum yield of photosynthesis in leaves in state 1. Biochim. Biophys. Acta 1837, 315–325 (2014).
Ballottari, M. et al. Regulation of photosystem I light harvesting by zeaxanthin. Proc. Natl Acad. Sci. USA 111, E2431–E2438 (2014).
Gobets, B. et al. Time-resolved fluorescence emission measurements of photosystem I particles of various cyanobacteria: a unified compartmental model. Biophys. J. 81, 407–424 (2001).
Wientjes, E., van Stokkum, I. H., van Amerongen, H. & Croce, R. The role of the individual Lhcas in photosystem I excitation energy trapping. Biophys. J. 101, 745–754 (2011).
Slavov, C., Ballottari, M., Morosinotto, T., Bassi, R. & Holzwarth, A. R. Trap-limited charge separation kinetics in higher plant photosystem I complexes. Biophys. J. 94, 3601–3612 (2008).
Jennings, R. C., Zucchelli, G., Croce, R. & Garlaschi, F. M. The photochemical trapping rate from red spectral states in PSI-LHCI is determined by thermal activation of energy transfer to bulk chlorophylls. Biochim. Biophys. Acta 1557, 91–98 (2003).
Benson, S. et al. An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nat. Plants 1, 15176 (2015).
Ganeteg, U., Külheim, C., Andersson, J. & Jansson, S. Is each light-harvesting complex protein important for plant fitness? Plant Physiol. 134, 502–509 (2004).
Ballottari, M., Mozzo, M., Croce, R., Morosinotto, T. & Bassi, R. Occupancy and functional architecture of the pigment binding sites of photosystem II antenna complex Lhcb5. J. Biol. Chem. 284, 8103–8113 (2009).
Cesaratto, A. et al. Analysis of cadmium-based pigments with time-resolved photoluminescence. Anal. Methods 6, 130–138 (2014).
van Stokkum, I. H., Larsen, D. S. & van Grondelle, R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta 1657, 82–104 (2004).
Bonente, G., Pippa, S., Castellano, S., Bassi, R. & Ballottari, M. Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J. Biol. Chem. 287, 5833–5847 (2012).
Acknowledgements
The work was financed by the Italian Ministry of Agriculture, Food and Forestry (MIPAAF) project HYDROBIO and by the Marie Curie Actions Initial Training Networks ACCLIPHOT (PITN-GA-2012-316427) and S2B (675006–SE2B) to R.B. G.C. acknowledges support from the European Research Council Advanced Grant STRATUS (ERC-2011-AdG No. 291198).
Author information
Authors and Affiliations
Contributions
M.Ba., L.D. and R.B. conceived the work and designed the experiments. M.Br. and L.D. performed all the experiments for the isolation of the ΔLhca mutant, its physiological and biochemical characterization and purification of PSI complexes. M.Ba. performed all the experiments for the spectroscopic characterization of PSI complexes. C.D. and G.C. coordinated the time-resolved fluorescence analysis experiments. I.B., M.J.P.A. and C.D. contributed to the time-resolved fluorescence analysis experiments. I.B., M.J.P.A., D.V., G.C., M.Ba. and C.D. analysed the fluorescence decay results by global analysis. All of the authors contributed to writing the manuscript. All of the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Supplementary Figs 1–11 and Supplementary References. (PDF 1849 kb)
Rights and permissions
About this article
Cite this article
Bressan, M., Dall'Osto, L., Bargigia, I. et al. LHCII can substitute for LHCI as an antenna for photosystem I but with reduced light-harvesting capacity. Nature Plants 2, 16131 (2016). https://doi.org/10.1038/nplants.2016.131
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2016.131
This article is cited by
-
A LHCB9-dependent photosystem I megacomplex induced under low light in Physcomitrella patens
Nature Plants (2018)
-
Loss of LHCI system affects LHCII re-distribution between thylakoid domains upon state transitions
Photosynthesis Research (2018)
-
Why is chlorophyll b only used in light-harvesting systems?
Journal of Plant Research (2018)
-
Photosynthesis: Complex flexibilities
Nature Plants (2016)