Abstract
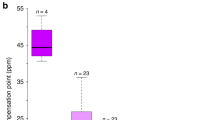
Ecogeographic rules explain spatial trends in biodiversity, species interactions and phenotypes1. Gloger's rule and its corollaries state that pigmentation of endothermic animals will increase from more polar to equatorial regions due to changing selective pressures including heat, humidity, predation and UV irradiance2–4. In plants, floral pigmentation varies within and among taxa, yet causes of wide-scale geographic variation are lacking. We show that Gloger's rule explains patterns of variation in UV-absorbing floral pigmentation in a widespread plant, Argentina anserina (Rosaceae). Specifically, the floral pigmentation pattern unique to the UV spectrum (UV ‘bullseye’) increases with proximity to the Equator in both hemispheres, and larger bullseyes are associated with higher UVB incidence. Experiments confirm UV as an agent of selection and bullseye size as a target. Results extend the generality of an ecogeographic rule—formulated for animals—to plants, implicating UV as a selective agent on a floral trait generally assumed to enhance plant–pollinator interactions. Global change is expected to alter UV irradiance in terrestrial systems5, potentially intensifying the importance of UV-mediated selection to floral evolution. Because floral UV reflectance and pattern enhance pollinator attraction6,7, altered selective regimes could disrupt coevolved plant–pollinator interactions, weakening an important ecosystem service8.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gaston, K. J., Chown, S. L. & Evans, K. L. Ecogeographical rules: elements of a synthesis. J. Biogeogr. 35, 483–500 (2008).
Gloger, C. L. Das Abändern der Vögel durch Einfluss der Klima's (A. Schulz, 1833).
Burtt, E. H. The adaptiveness of animal colors. BioScience 31, 723–729 (1981).
Caro, T. The adaptive significance of coloration in mammals. BioScience 55, 125–136 (2005).
Ballaré, C. L., Caldwell, M. M., Flint, S. D., Robinson, S. A. & Bornman, J. F. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem. Photobiol. Sci. 10, 226–241 (2011).
Johnson, S. D. & Andersson, S. A simple field method for manipulating ultraviolet reflectance of flowers. Can. J. Bot. 80, 1325–1328 (2002).
Koski, M. H. & Ashman, T.-L. Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Funct. Ecol. 28, 868–877 (2014).
Costanza, R. et al. The value of the world's ecosystem services and natural capital. Nature 387, 253–260 (1997).
Jablonski, N. G. & Chaplin, G. Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 107, 8962–8968 (2010).
Santana, S. E., Lynch Alfaro, J. & Alfaro, M. E. Adaptive evolution of facial colour patterns in Neotropical primates. Proc. R. Soc. B Biol. Sci. 279, 2204–2211 (2012).
Hamada, Y., Suryobroto, B., Goto, S. & Malaivijitnond, S. Morphological and body color variation in Thai Macaca fascicularis North and South of the Isthmus of Kra. Int. J. Primatol. 29, 1271–1294 (2008).
Lai, Y.-C., Shiroishi, T., Moriwaki, K., Motokawa, M. & Yu, H.-T. Variation of coat color in house mice throughout Asia. J. Zool. 274, 270–276 (2008).
Millien, V. et al. Ecotypic variation in the context of global climate change: revisiting the rules. Ecol. Lett. 9, 853–869 (2006).
Guldberg, L. D. & Atsatt, P. R. Frequency of reflection and absorption of ultraviolet light in flowering plants. Am. Midl. Nat. 93, 35–43 (1975).
Harborne, J. B. & Nash, R. J. Flavonoid pigments responsible for ultraviolet patterning in petals of the genus Potentilla. Biochem. Syst. Ecol. 12, 315–318 (1984).
Gorton, H. L. & Vogelmann, T. C. Effects of epidermal cell shape and pigmentation on optical properties of Antirrhinum petals at visible and ultraviolet wavelengths. Plant Physiol. 112, 879–888 (1996).
Rausher, M. D. Evolutionary transitions in floral color. Int. J. Plant Sci. 169, 7–21 (2008).
Whittall, J. B. & Strauss, S. in Ecology and Evolution of Flowers 120–138 (Oxford Univ. Press, 2006).
Torabinejad, J. M., Caldwell, M., Flint, S. A. & Durham, S. Susceptibility of pollen to UV-B radiation: an assay of 35 taxa. Am. J. Bot. 84, 360–369 (1998).
Koti, S. Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination, and tube lengths. J. Exp. Bot. 56, 725–736 (2005).
Herman, J. R. Global increase in UV irradiance during the past 30 years (1979–2008) estimated from satellite data. J. Geophys. Res. 115, D04203 (2010).
Yoshioka, Y. et al. Intraspecific variation in the ultraviolet colour proportion of flowers in Brassica rapa L. Plant Breed. 124, 551–556 (2005).
Koski, M. H. & Ashman, T.-L. Quantitative variation, heritability, and trait correlations for ultraviolet floral traits in Argentina anserina (Rosaceae): Implications for floral evolution. Int. J. Plant Sci. 174, 1109–1120 (2013).
Oksanen, J. et al. vegan: Community Ecology Package (2013); http://cran.r-project.org/web/packages/vegan/index.html.
Schluter, D. Estimating the form of natural selection on a quantitative trait. Evolution 42, 849–861 (1988).
Conner, J. K., Rice, A. M., Stewart, C. & Morgan, M. T. Patterns and mechanisms of selection on a family-diagnostic trait: evidence from experimental manipulation and lifetime fitness selection gradients. Evolution 57, 480–486 (2003).
Jablonski, N. The evolution of human skin coloration. J. Hum. Evol. 39, 57–106 (2000).
Arista, M., Talavera, M., Berjano, R. & Ortiz, P. L. Abiotic factors may explain the geographical distribution of flower colour morphs and the maintenance of colour polymorphism in the scarlet pimpernel. J. Ecol. 101, 1613–1622 (2013).
Zhang, C., Yang, Y.-P. & Duan, Y.-W. Pollen sensitivity to ultraviolet-B (UV-B) suggests floral structure evolution in alpine plants. Sci. Rep. 4, 4520 (2014).
Dupont, Y. L., Padrón, B., Olesen, J. M. & Petanidou, T. Spatio-temporal variation in the structure of pollination networks. Oikos 118, 1261–1269 (2009).
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005).
Beckmann, M. et al. glUV: A global UV-B radiation dataset for macroecological studies. Methods Ecol. Evol. 5, 372–383 (2014).
Lande, R. & Arnold, S. J. The measurement of selection on correlated characters. Evolution 37, 1210–1226 (1983).
Etterson, J. R. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. I. Clinal patterns of selection along an environmental gradient in the great plains. Evolution 58, 1446–1456 (2004).
Acknowledgements
We thank J. Reithel and A. Robertson for logistical support, S. Barratt-Boyes, G. Arceo-Gomez, T. Knight, S.D. Smith and Ashman Lab members for discussion, BLM and USFS for access to populations, N. Morehouse for access to spectrophotometers and A.M. Koski, L.J. Koski and T.M. Byers for field assistance. Funding was provided by grants from SSE, BSA, Sigma-Xi, RMBL and National Geographic to M.H.K., NSF DEB 1020523 and 1241006 to T-L.A. and NSF DEB 1309450 to M.H.K. and T-L.A. M.H.K. was supported by a NSF GRFP and UPitt Mellon Fellowship.
Author information
Authors and Affiliations
Contributions
M.H.K. and T-L.A. designed the research; M.H.K. performed experiments and analyses; M.H.K. and T-L.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Koski, M., Ashman, TL. Floral pigmentation patterns provide an example of Gloger's rule in plants. Nature Plants 1, 14007 (2015). https://doi.org/10.1038/nplants.2014.7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2014.7
This article is cited by
-
Nectar mimicry: a new phenomenon
Scientific Reports (2020)
-
Genetic diversity patterns in ex situ collections of Oryza officinalis Wall. ex G. Watt revealed by morphological and microsatellite markers
Genetic Resources and Crop Evolution (2017)
-
Flower colour: Gloger's rule isn't just for the birds
Nature Plants (2015)