Abstract
Bulk water has three phases: solid, liquid and vapour. In addition to undergoing a phase transition (of the first order) between them, liquid and vapour can deform continuously into each other without crossing a transition line—in other words, there is no intrinsic distinction between the two phases. Hence, the first-order line of the liquid–vapour phase transition should terminate at a critical point. In contrast, the first-order transition line between solid and liquid is believed to persist indefinitely without terminating at a critical point1. In recent years, however, it was reported that inside carbon nanotubes, freezing of water may occur continuously as well as discontinuously through a first-order phase transition2. Here we present simulation results for water in a quasi-two-dimensional hydrophobic nanopore slit, which are consistent with the idea that water may freeze by means of both first-order and continuous phase transitions. Our results lead us to hypothesize the existence of a connection point at which first-order and continuous transition lines meet3,4.
Similar content being viewed by others
Main
It is widely believed that a solid–liquid phase transition line does not terminate at a critical point, although it has not been possible to prove rigorously the non-existence of the solid–liquid critical point1. On the other hand, after the discovery of carbon nanotubes5, a solid–liquid phase transition inside these structures has been reported, together with the intriguing possibility of a solid–liquid critical point, presumably because of the constraints in packing inside carbon nanotubes6. In recent years, therefore, there has been a great deal of interest in the simulation report that freezing of water inside carbon nanotubes may occur continuously as well as discontinuously through a first-order transition2,6,7.
As for the solid–liquid phase transition, two-dimensional systems are of particular interest. According to the Mermin–Wagner theorem, continuous symmetry in two dimensions cannot be broken at non-zero temperatures, which means that there is no true long-ranged crystalline order in two-dimensional systems8. However, this does not necessarily rule out the presence of a phase transition; instead the Kosterlitz–Thouless–Halperin–Nelson–Young (KTHNY) theory states that melting in two dimensions may involve two continuous transitions, one from the solid phase to a hexatic phase and the other from the hexatic phase to the liquid phase9. Although the KTHNY theory seems rather appealing and general, it is still controversial whether the KTHNY theory provides the only mechanism of the melting in two dimensions9,10. Here we present simulation results supporting the possibility that a solid–liquid phase transition of water in quasi-two-dimensions may occur through both first-order and continuous phase transitions, depending on the density or pressure.
We carry out extensive molecular dynamics simulations of the TIP5P model11 of N=256 water molecules confined between two unstructured and smooth hydrophobic plates (see ref. 12 for detailed simulation descriptions). We choose the separation H=0.8 nm between two parallel (L×L) plates to contain maximally two layers of water molecules, so that confinement effects on water are extremely strong. We use the linear size of the plate from L=32.01 Å up to L=42.70 Å, depending on the density ρ. The water–wall interactions are modelled by a 9–3 Lennard-Jones potential, which is commonly used to represent the effective interactions of water molecules with the confining surfaces13,14. In molecular dynamics simulations we adopt the constant molecule number, volume and temperature (N V T) ensemble with periodic boundary conditions in the parallel (x and y) directions, and analyse wide ranges of ρ from 0.81 g cm−3 up to 1.45 g cm−3 and the temperature T from 300 K down to 230 K. We carry out molecular dynamics simulations for 10–60 ns, depending on T and ρ. To confirm the results of N V T ensemble simulations, we also carry out molecular dynamics simulations on the N Px yT ensemble, where Px y is the pressure in the direction parallel to the plates. We present the results of N Px yT ensemble simulations in the Supplementary Information.
Figure 1 shows the potential energy U as a function of T for various densities. For a range of constant density paths from ρ=0.99 g cm−3 up to 1.28 g cm−3, as T decreases, we find large drops in U (5–6 kJ mol−1). The temperature range in which the drop occurs depends on the density. Such a sudden drop in U is consistent with the presence of a first-order phase transition between liquid and solid at the temperature T0 of the drop15,16. For ρ≥1.33 g cm−3, on the other hand, the sharp drop suddenly disappears and U decreases gradually as T is lowered to 230 K. It is of interest to compare the two cases, ρ=1.33 g cm−3 and 1.30 g cm−3. Whereas the two exhibit the same behaviour at temperatures T=300 K down to 260 K, different behaviours begin to develop for T≤250 K. For a continuous drop of U for ρ≥1.33 g cm−3, there are three possible explanations. One is that water remains in the liquid state from T=300 K down to 230 K, so that there is no sudden drop in U. Another is the existence of a weak first-order phase transition between liquid and solid, where the drop is too small to measure in our simulations. The third is the presence of a continuous phase transition at some temperature between 300 K and 230 K. In the last two cases there would exist a critical density ρc between 1.30 g cm−3 and 1.33 g cm−3, above which the phase transition becomes (quasi-) continuous.
a,b, Potential energy U as a function of temperature T for densities ρ=1.30 g cm−3 and below (a) and densities ρ=1.33 g cm−3 and above (b). At density ρ=1.16 g cm−3, on cooling from T=300 K, there is a sudden drop (∼6 kJ mol−1) in U across the transition temperature T0 between 280 K and 270 K, which manifests a first-order phase transition between solid and liquid. Such transition behaviour persists up to ρ=1.30 g cm−3, although the drop in U is reduced (∼3 kJ mol−1) with its location T0 depending on the density. When ρ reaches 1.33 g cm−3, however, there is no sharp drop and U decreases gradually as T is lowered down to 230 K. We thus suggest that as the density is raised, the nature of the phase transition might change from a first-order transition to a continuous one at the critical density ρc, located between 1.30 g cm−3 and 1.33 g cm−3.
To determine which scenario is correct, we first consider the lateral oxygen–oxygen radial distribution function (RDF) gOOxy versus the lateral position rx y parallel to the confining plates17, and examine explicitly how the structure of water in the parallel direction changes. For ρ=1.28 g cm−3, we observe characteristic features of liquid and of solid in Fig. 2 at high and at low temperatures, respectively. At T=280 K, the RDF exhibits typical liquid behaviour, which consists of a pre-peak representing a bilayer, and the prominent first peak at rx y=0.28 nm, followed by rapidly decaying peaks and the eventual approach to unity indicating the disordered state12. At T=270 K, on the other hand, the RDF exhibits long-ranged order, showing that water has transformed to ice. The behaviour of the RDF, characteristic of a first-order transition1, changes abruptly when the temperature is varied across the transition temperature T0 between 270 K and 280 K. For the higher density ρ=1.33 g cm−3, the RDF at low temperatures also shows long-ranged order, again corresponding to ice. However, the temperature dependence of the RDF exhibits behaviour different from that in the case of lower densities. Instead of the abrupt disappearance of the long-ranged order observed for ρ=1.28 g cm−3, the long-ranged order gradually reduces as the temperature is raised. Such gradual (rather than abrupt) variation of the RDF with temperature, which persists at higher densities (ρ≥1.33 g cm−3>ρc), illustrates that the phase changes continuously, in contrast with the case of lower densities (ρ≤1.30 g cm−3<ρc) exhibiting a discontinuous phase change. Therefore, the RDF results suggest that the nature of the phase transition between solid and liquid changes across the critical density ρ=ρc, where 1.30 g cm−3<ρc<1.33 g cm−3. Note that ice formations for ρ<ρc and ρ>ρc are different. For ρ>ρc ice structure is rhombic, whereas for ρ<ρc it is hexagonal (Fig. 3). During slow cooling and heating processes, we also find that U shows a hysteresis loop for ρ<ρc but rather a continuous change without a hysteresis loop for ρ>ρc, supporting two different phase transitions (see the Supplementary Information).
a, The RDF at density ρ=1.28 g cm−3 (<ρc) exhibits characteristic features of liquids and of solids, respectively, depending on temperature T. When T is varied across 270 K and 280 K, the RDF changes abruptly, indicating a first-order transition between liquid and solid. b, The RDF at ρ=1.33 g cm−3 (>ρc), unlike that for ρ<ρc, varies continuously rather than abruptly over the entire temperature range. At T=230 K, it exhibits long-range order, corresponding to the crystalline order. As T is raised, the long-range order of the RDF becomes faint, turning into liquid-like disorder. c, The TDP at ρ=1.28 g cm−3 also exhibits the abrupt change of the structure seen for the RDF at the same temperature between 270 K and 280 K. This indicates that the structural change occurs discontinuously in the direction both parallel and perpendicular to the confining surfaces. d, At ρ=1.33 g cm−3, the TDP varies gradually with T, which is again consistent with the structural change observed in the RDF. Accordingly, behaviours of both the RDF and the TDP support the scenario that the phase transition between liquid and solid changes from a first-order transition to a continuous one, as ρ is increased above ρc.
To confirm the presence of two different solid–liquid phase transitions, we next examine the structural change of water in the transverse (confining) direction. The difference in the structural behaviour for ρ<ρc and for ρ>ρc is revealed in the transverse density profile (TDP) ρz versus the confining coordinate z (ref. 17). In Fig. 2, the TDP for ρ=1.28 g cm−3 (<ρc) exhibits big jumps in the two peaks at T≈T0 between 270 K and 280 K. Namely, the structure in the direction perpendicular to the confining surfaces also changes discontinuously at T=T0. For ρ=1.33 g cm−3 (>ρc), on the other hand, the two peaks of the TDP do not show any appreciable jumps with temperature; instead the TDP varies gradually with T, similar to the RDF for ρ>ρc. These consistent behaviours of the RDF and the TDP support our hypothesis that the nature of the phase transition between solid and liquid changes as ρ is varied across ρc. From the N Px yT simulations for Px y<Pc and Px y>Pc (where Pc is the critical lateral pressure), we find that ρ changes discontinuously by crossing a transition line for Px y<Pc. In contrast, it changes continuously for Px y>Pc, manifesting two different phase transitions (see the Supplementary Information).
We further probe the dynamical properties for ρ>ρc, which are conveniently characterized by the lateral mean-square displacement (MSD) Δx y(t)≡〈[rx y(t)−rx y(0)]2〉 (ref. 17). Figure 4 shows the mobility difference between liquid and solid, measured by the MSD as a function of time t, for density ρ=1.33 g cm−3 at various temperatures. At T=300 K, the MSD, starting initially from the ballistic regime (∝t2), finally reaches the diffusive regime (∝t). This behaviour persists down to T=250 K, which suggests a liquid phase, although the slope of the MSD continues to decrease as T is lowered. At T=240 K, the MSD remains almost flat (constant) with time, indicating that water has frozen to ice. Although the mobility at temperatures from 300 K down to 250 K is distinguishable from that at T=240 K, it varies in a continuous way; the slope of the MSD for the liquid approaches continuously the slope for the solid. Such continuity is not observed in the case of a first-order transition. It has been shown that the diffusion constant D, which is related to the slope of MSD through Einstein’s relation17, changes abruptly when the first-order transition line is crossed18. For ice, D∼10−10 cm2 s−1, whereas for liquid water D∼10−5 cm2 s−1 (refs 2, 12, 16, 18, 19). Therefore, the mobility can serve as a good measure of a phase transition20 and provides further evidence for the existence of both the solid and the liquid phases separated by a (continuous) phase transition for ρ>ρc. Such continuous freezing has been experimentally observed in diffraction measurements of water inside cylindrical nanopores21.
At temperatures T=300 K down to 250 K, the MSD, starting from the ballistic regime (∝t2), eventually reaches the diffusive regime (∝t) in the long time t. The plateau between the two regimes, observed at low temperatures, arises from the cage effect. We thus conclude that the MSD exhibits typical liquid behaviour. At T=240 K, in contrast, the evolution of the MSD is almost flat and exhibits large fluctuations, indicating that water has turned into ice. The diffusion constant D, calculated from the MSD, takes the values 5.7×10−6 cm2 s−1, 1.3×10−6 cm2 s−1 and 2.2×10−7 cm2 s−1 at T=300 K, 270 K and 250 K, respectively, whereas at T=240 K it is given by 5.2×10−9 cm2 s−1.
In addition to the well-known liquid–vapour critical point, another critical point of the liquid–liquid phase transition has been found in simulations of water22. As for phases of solid and liquid, however, a symmetry argument forbids the presence of a solid–liquid critical point in bulk water1. For confined water, noteworthy in the limit of high density are the layering effects, which arise from confining boundary conditions17,23. Layering, observed in the TDP for high densities, in general hinders water molecules from leaving the layer and facilitates them to form a relatively ordered structure compared with typical liquid water. As T is subsequently lowered, water is expected to freeze without changing its relatively ordered structure, so the structure of liquid water should be similar to that of ice2. As a quasi-two-dimensional system, monolayer water may not be expected to show a continuous transition at high pressures, because the freezing of monolayer water at high pressures is generally coupled to a first-order buckling transition12,18.
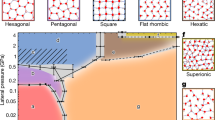
In the case of water in a quasi-two-dimensional hydrophobic nanopore slit, we have found that the first-order transition line may connect with a continuous transition line (see Fig. 5), instead of terminating at the critical point. The resulting connection point between the two transition lines is located at 1.30 g cm−3<ρc<1.33 g cm−3 and 240 K<Tc<250 K. This is in contrast to the quasi-one-dimensional system of water inside carbon nanotubes, where the first-order transition line terminates at a solid–liquid critical point2, and beyond the point it seems that one phase can deform continuously to the other without crossing a transition line, similarly to the case of a liquid–vapour critical point1,2. Even in three dimensions there is a simulation result reporting evidence for a solid–liquid critical point in a simple monatomic system24. Recall that the mobility of water molecules presented in Fig. 4 seems supportive of the existence of a continuous phase transition at high densities. Such a continuous transition was also observed between the liquid and the hexatic phase in a monolayer of Lennard-Jones fluids inside a quasi-two-dimensional pore slit25. In addition, simulations of a two-dimensional system with short-range attractive interactions revealed a solid–solid critical point that terminates a first-order transition line between two solids26.
Whereas at low densities confined water undergoes a phase transition between solid (green circles) and liquid (yellow circles) phases by crossing a first-order line, the phase changes rather continuously for ρ exceeding ρc. Accordingly, the first-order transition line (represented by the black solid line) continues to a continuous transition line (the blue dashed line), and there is a connection point between two transition lines (the red bullet). The connection point is located in the ranges of density 1.30 g cm−3<ρc<1.33 g cm−3 and of temperature 240 K<Tc<250 K, whereas the location of a transition line has an uncertainty ΔT∼±5 K and Δρ∼±0.05 g cm−3. In the ρ–T phase diagram, a first-order phase transition should be represented by a region of the two-phase coexistence. However, the finite size of the system and the choice of the N V T ensemble apparently lead the coexistence region to be narrow, so that the coexistence region here appears as a line. Here, ρ in the N V T ensemble plays almost the same role as P in the N P T ensemble. Two ice phases have different symmetries and are thus separated by a phase boundary (denoted by a green solid line).
Whereas for liquid water unstructured walls in general reproduce the same results as atomic and structured walls27, this is not clear for a solid–liquid phase transition. In view of the fact that the phase transition in confined water is surface-induced28, it is likely that the nature of confining walls should affect the freezing mechanism of water. In particular, in the search for a continuous solid–liquid phase transition, hydrophobicity of surfaces might be crucial, as both in experiments29 and in simulations16 freezing has not been observed for water confined in hydrophilic environments. Although our finding is consistent with a number of existing results in (quasi-)two dimensions, we do not exclude the possibility of first-order phase transitions at high densities rounded off by finite-size effects. Concerning experimental evidence, the recent report of ice at room temperature formed from nanoconfined water30, as well as the measurement of water inside nanopores21, is encouraging.
References
Stanley, H. E. Introduction to Phase Transitions and Critical Phenomena (Oxford Univ. Press, 1971).
Koga, K., Gao, G. T., Tanaka, H. & Zeng, X. C. Formation of ordered ice nanotubes inside carbon nanotubes. Nature 412, 802–805 (2001).
Landau, L. D. & Lifshitz, E. M. Statistical Physics: Part 1 (Butterworth–Heinemann, 2001).
Griffiths, R. B. Thermodynamics near the two-fluid critical mixing point in He3–He4. Phys. Rev. Lett. 24, 715–717 (1970).
Iijima, S. Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991).
Ball, P. New horizons in inner space. Nature 361, 297 (1993).
Koga, K. Freezing in one-dimensional liquids. J. Chem. Phys. 118, 7973–7980 (2003).
Mermin, N. D. Crystalline order in two dimensions. Phys. Rev. 176, 250–254 (1968).
Strandburg, K. J. Two-dimensional melting. Rev. Mod. Phys. 60, 161–207 (1988).
Dash, J. G. History of the search for continuous melting. Rev. Mod. Phys. 71, 1737–1743 (1999).
Mahoney, M. W. & Jorgensen, W. L. A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J. Chem. Phys. 112, 8910–8922 (2000).
Kumar, P., Buldyrev, S. V., Starr, F. W., Giovambattista, N. & Stanley, H. E. Thermodynamics, structure, and dynamics of water confined between hydrophobic plates. Phys. Rev. E 72, 051503 (2005).
Steele, W. A. The Interaction of Gases with Solid Surfaces (Oxford, 1974).
Han, S., Kumar, P. & Stanley, H. E. Hydrogen-bond dynamics of water in a quasi-two-dimensional hydrophobic nanopore slit. Phys. Rev. E 79, 041202 (2009).
Koga, K., Zeng, X. C. & Tanaka, H. Freezing of confined water: A bilayer ice phase in hydrophobic nanopores. Phys. Rev. Lett. 79, 5262–5265 (1997).
Koga, K., Tanaka, H. & Zeng, X. C. First-order transition in confined water between high-density liquid and low-density amorphous phases. Nature 408, 564–567 (2000).
Hansen, J-P. & McDonald, I. R. Theory of Simple Liquids (Academic, 2006).
Zangi, R. Water confined to a slab geometry: A review of recent computer simulation studies. J. Phys. Condens. Matter 16, S5371–S5388 (2004).
Han, S., Kumar, P. & Stanley, H. E. Absence of a diffusion anomaly of water in the direction perpendicular to hydrophobic nanoconfining walls. Phys. Rev. E 77, 030201(R) (2008).
Hedges, L. O., Jack, R. L., Garrahan, J. P. & Chandler, D. Dynamic order–disorder in atomistic models of structural glass formers. Science 323, 1309–1313 (2009).
Morishige, K. & Kawano, K. Freezing and melting of water in a single cylindrical pore: The pore-size dependence of freezing and melting behaviour. J. Chem. Phys. 110, 4867–4872 (1999).
Poole, P. H., Sciortino, F., Essmann, U. & Stanley, H. E. Phase behaviour of metastable water. Nature 360, 324–328 (1992).
Gao, J., Luedtke, W. D. & Landman, U. Layering transitions and dynamics of confined liquid films. Phys. Rev. Lett. 79, 705–708 (1997).
Elenius, M. & Dzugutov, M. Evidence for a liquid–solid critical point in a simple monatomic system. J. Chem. Phys. 131, 104502 (2009).
Radhakrishnan, R., Gubbins, K. E. & Sliwinska-Bartkowiak, M. Existence of a hexatic phase in porous media. Phys. Rev. Lett. 89, 076101 (2002).
Bladon, P. & Frenkel, D. Dislocation unbinding in dense two-dimensional crystals. Phys. Rev. Lett. 74, 2519–2522 (1995).
Lee, S. H. & Rossky, P. J. A comparison of the structure and dynamics of liquid water at hydrophobic and hydrophilic surfaces—a molecular dynamics simulation study. J. Chem. Phys. 100, 3334–3345 (1994).
Giovambattista, N., Rossky, P. J. & Debenedetti, P. G. Phase transitions induced by nanoconfinement in liquid water. Phys. Rev. Lett. 102, 050603 (2009).
Raviv, U., Laurat, P. & Klein, P. J. Fluidity of water confined to subnanometre films. Nature 413, 51–54 (2001).
Jinesh, K. B. & Frenken, J. W. M. Experimental evidence for ice formation at room temperature. Phys. Rev. Lett. 101, 036101 (2008).
Acknowledgements
We thank S. V. Buldyrev for helpful discussions. S.H., P.K. and H.E.S. acknowledge support from NSF Grant No. CHE 0606489 and the Keck Foundation NAKFI award. M.Y.C. acknowledges support from NRF through the Basic Science Research Program (2009–0080791).
Author information
Authors and Affiliations
Contributions
S.H., M.Y.C., P.K. and H.E.S. designed the research; S.H. carried out simulations; and S.H., M.Y.C., P.K. and H.E.S. analysed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 319 kb)
Rights and permissions
About this article
Cite this article
Han, S., Choi, M., Kumar, P. et al. Phase transitions in confined water nanofilms. Nature Phys 6, 685–689 (2010). https://doi.org/10.1038/nphys1708
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys1708
This article is cited by
-
Wettability and confinement size effects on stability of water conveying nanotubes
Scientific Reports (2020)
-
Observation of an exotic state of water in the hydrophilic nanospace of porous coordination polymers
Communications Chemistry (2020)
-
Real-space visualization of intercalated water phases at the hydrophobic graphene interface with atomic force microscopy
Frontiers of Physics (2020)
-
Fluidity and phase transitions of water in hydrophobic and hydrophilic nanotubes
Scientific Reports (2019)
-
Structure and dynamics in the lithium solvation shell of nonaqueous electrolytes
Scientific Reports (2019)