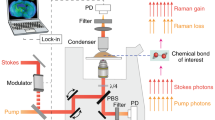
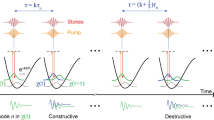
Abstract
Raman scattering provides an intrinsic fingerprint of chemical composition. Spontaneous Raman spectroscopy has been used for many decades to interrogate biological materials and systems. In spite of its valuable information content, Raman imaging is rarely used compared with modalities such as fluorescence microscopy because of its relatively slow signal acquisition. Coherent Raman imaging technologies have evolved over the past fifteen years to now capture rich chemical information with improved acquisition speeds. As a result, coherent Raman imaging methods are now poised to begin emerging as widely used tools for obtaining functional information in a label-free manner from biological systems. We briefly review the development and application of these methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chan, J. W. & Lieu, D. K. Label-free biochemical characterization of stem cells using vibrational spectroscopy. J. Biophoton. 2, 656–668 (2009).
Notingher, I. et al. In situ spectral monitoring of mRNA translation in embryonic stem cells during differentiation in vitro. Anal. Chem. 76, 3185–3193 (2004).
Notingher, I., Jell, G., Lohbauer, U., Salih, V. & Hench, L. L. In situ non-invasive spectral discrimination between bone cell phenotypes used in tissue engineering. J. Cell. Biochem. 92, 1180–1192 (2004).
Bakker Schut, T. C., Wolthuis, R., Caspers, P. J. & Puppels, G. J. Real-time tissue characterization on the basis of in vivo Raman spectra. J. Raman Spectrosc. 33, 580–585 (2002).
Gentleman, E. et al. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nature Mater. 8, 763–770 (2009).
Gniadecka, M. et al. Melanoma diagnosis by Raman spectroscopy and neural networks: structure alterations in proteins and lipids in intact cancer tissue. J. Invest. Dermatol. 122, 443–449 (2004).
Haka, A. S. et al. Diagnosing breast cancer by using Raman spectroscopy. Proc. Natl Acad. Sci. USA 102, 12371–12376 (2005).
Krafft, C., Sobottka, S. B., Schackert, G. & Salzer, R. Near infrared Raman spectroscopic mapping of native brain tissue and intracranial tumors. Analyst 130, 1070–1077 (2005).
Krafft, C., Sobottka, S. B., Schackert, G. & Salzer, R. Raman and infrared spectroscopic mapping of human primary intracranial tumors: a comparative study. J. Raman Spectrosc. 37, 367–375 (2006).
Pestov, D. et al. Coherent versus incoherent Raman scattering: molecular coherence excitation and measurement. Opt. Lett. 32, 1725–1727 (2007).
Saar, B. G. et al. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330, 1368–1370 (2010).
Evans, C. L. et al. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl Acad. Sci. USA 102, 16807–16812 (2005).
Camp, C. H. Jr et al. High-speed coherent Raman fingerprint imaging of biological tissues. Nature Photon. 8, 627–634 (2014).
Raman, C. V. A new radiation. Indian J. Phys. 398, 368–376 (1928).
Raman, C. V. & Krishnan, K. S. A new type of secondary radiation. Nature 121, 501–502 (1928).
Smekal, A. Zur Quantentheorie der Dispersion. Naturwissenschaften 11, 873–875 (1923).
Landsberg, G. & Mandelstam, L. Eine neue Erscheinung bei der Lichtzerstreuung in Krystallen. Naturwissenschaften 16, 557–558 (1928).
Schawlow, A. & Townes, C. Infrared and optical masers. Phys. Rev. 112, 1940–1949 (1958).
Maiman, T. H. Stimulated optical radiation in ruby. Nature 187, 493–494 (1960).
Denson, S. C., Pommier, C. J. S. & Denton, M. B. The impact of array detectors on Raman spectroscopy. J. Chem. Educ. 84, 67–74 (2007).
Adar, F., Division, R. S., Ave, P. & Yvon, H. J. Evolution of instrumentation for detection of the Raman Effect as driven by available technologies and by developing applications. J. Chem. Educ. 84, 50–60 (2007).
Koenig, J. L. Raman spectroscopy of biological molecules: A review. J. Polym. Sci. Macromol. Rev. 6, 59–177 (1972).
Bergholt, M. S. et al. Characterizing variability in in vivo Raman spectra of different anatomical locations in the upper gastrointestinal tract toward cancer detection. J. Biomed. Opt. 16, 037003 (2011).
Bergholt, M. S. Raman endoscopy for objective diagnosis of early cancer in the gastrointestinal system. J. Gastrointest. Dig. Syst. http://doi.org/3ns (2013).
Bergholt, M. S. et al. Fiberoptic confocal raman spectroscopy for real-time in vivo diagnosis of dysplasia in Barrett's esophagus. Gastroenterology 146, 27–32 (2014).
Lieber, C. A., Majumder, S. K., Ellis, D. L., Billheimer, D. D. & Mahadevan-Jansen, A. In vivo nonmelanoma skin cancer diagnosis using Raman microspectroscopy. Lasers Surg. Med. 40, 461–467 (2008).
Nijssen, A. et al. Discriminating basal cell carcinoma from its surrounding tissue by Raman spectroscopy. J. Invest. Dermatol. 119, 64–69 (2002).
Motz, J. T. et al. Real-time Raman system for in vivo disease diagnosis. J. Biomed. Opt. 10, 031113 (2005).
Zumbusch, A., Holtom, G. & Xie, X. Three-dimensional vibrational imaging by coherent anti-Stokes Raman scattering. Phys. Rev. Lett. 82, 4142–4145 (1999).
Duncan, M. D., Reintjes, J. & Manuccia, T. J. Scanning coherent anti-Stokes Raman microscope. Opt. Lett. 7, 350–352 (1982).
Nandakumar, P., Kovalev, A. & Volkmer, A. Vibrational imaging based on stimulated Raman scattering microscopy. New J. Phys. 11, 033026 (2009).
Ozeki, Y., Dake, F., Kajiyama, S., Fukui, K. & Itoh, K. Analysis and experimental assessment of the sensitivity of stimulated Raman scattering microscopy. Opt. Express 17, 3651–3658 (2009).
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Ploetz, E., Laimgruber, S., Berner, S., Zinth, W. & Gilch, P. Femtosecond stimulated Raman microscopy. Appl. Phys. B 87, 389–393 (2007).
Cheng, J-X., Volkmer, A., Book, L. D. & Xie, X. S. Multiplex coherent anti-Stokes Raman scattering microspectroscopy and study of lipid vesicles. J. Phys. Chem. B 106, 8493–8498 (2002).
Müller, M. & Schins, J. M. Imaging the thermodynamic state of lipid membranes with multiplex CARS microscopy. J. Phys. Chem. B 106, 3715–3723 (2002).
Kee, T. W. & Cicerone, M. T. Simple approach to one-laser, broadband coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 29, 2701–2703 (2004).
Kano, H. & Hamaguchi, H. Ultrabroadband (>2500 cm−1) multiplex coherent anti-Stokes Raman scattering microspectroscopy using a supercontinuum generated from a photonic crystal fiber. Appl. Phys. Lett. 86, 121113 (2005).
Volkmer, A., Book, L. D. & Xie, X. S. Time-resolved coherent anti-Stokes Raman scattering microscopy: imaging based on Raman free induction decay. Appl. Phys. Lett. 80, 1505–1507 (2002).
Ogilvie, J. P., Beaurepaire, E., Alexandrou, A. & Joffre, M. Fourier-transform coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 31, 480–482 (2006).
Cui, M., Joffre, M., Skodack, J. & Ogilvie, J. P. Interferometric Fourier transform coherent anti-Stokes Raman scattering. Opt. Express 14, 8448–8458 (2006).
Marks, D. & Boppart, S. A. Nonlinear interferometric vibrational imaging. Phys. Rev. Lett. 92, 123905 (2004).
Jiang, Z., Marks, D. L., Geddes, J. B. III & Boppart, S. A. Nonlinear interferometric vibrational imaging of biological tissue. Proc. SPIE 6860, 68600Y (2008).
Ideguchi, T. et al. Coherent Raman spectro-imaging with laser frequency combs. Nature 502, 355–358 (2013).
Dudovich, N., Oron, D. & Silberberg, Y. Single-pulse coherently controlled nonlinear Raman spectroscopy and microscopy. Nature 418, 512–514 (2002).
Bachler, B. R., Fermann, M. E. & Ogilvie, J. P. Multiplex Raman induced Kerr effect microscopy. Opt. Express 20, 835–844 (2012).
Kumar, V. et al. Balanced-detection Raman-induced Kerr-effect spectroscopy. Phys. Rev. A 86, 053810 (2012).
Freudiger, C. W. et al. Optical heterodyne-detected Raman-induced Kerr effect (OHD-RIKE) microscopy. J. Phys. Chem. B 115, 5574–5581 (2011).
Rock, W., Bonn, M. & Parekh, S. H. S. Near shot-noise limited hyperspectral stimulated Raman scattering spectroscopy using low energy lasers and a fast CMOS array. Opt. Express 21, 15113–15120 (2013).
Zhang, X. et al. Label-free live-cell imaging of nucleic acids using stimulated Raman scattering microscopy. ChemPhysChem 13, 1054–1059 (2012).
Agrawal, G. P. Nonlinear Fiber Optics (Academic, 2001).
Boyd, R. W. Nonlinear Optics (Academic, 2003).
Cheng, J-X., Volkmer, A. & Xie, X. S. Theoretical and experimental characterization of coherent anti-Stokes Raman scattering microscopy. J. Opt. Soc. Am. B 19, 1363 (2002).
Potma, E. O. & Mukamel, S. in Coherent Raman Scattering Microscopy (eds Cheng, J-X. & Xie, X. S.) 3–42 (CRC, 2013).
Hellwarth, R. W. Third-order optical susceptibilities of liquids and solids. Prog. Quant. Electron. 5, 1–68 (1979).
Konigstein, J. A. Introduction to the Theory of the Raman Effect (D Reidel, 1972).
Long, D. A. Raman Spectroscopy (McGraw-Hill, 1977).
Mukamel, S. Principles of Nonlinear Optical Spectroscopy (Oxford Univ. Press, 1995).
Tolles, W. M., Nibler, J. W., Mcdonald, J. R. & Harvey, A. B. A review of the theory and application of coherent anti-Stokes Raman spectroscopy (CARS). Appl. Spectrosc. 31, 253–271 (1977).
Potma, E. O., Xie, X. S., Volkmer, A. & Cheng, J-X. in Coherent Raman Scattering Microscopy (eds Cheng, J-X. & Xie, X. S.) 43–78 (CRC, 2013).
Stevenson, T. L. & Vo-Dinh, T. in Modern Techniques in Raman Spectroscopy (ed. Laserna, J. J.) 1–40 (John Wiley & Sons, 1996).
Gomez, J. S. in Modern Techniques in Raman Spectroscopy (ed. Laserna, J. J.) 305–342 (John Wiley & Sons, 1996).
Zhang, D. et al. Spectrally modulated stimulated Raman scattering imaging with an angle-to-wavelength pulse shaper. Opt. Express 21, 2641–2643 (2013).
Berto, P., Andresen, E. R. & Rigneault, H. Background-free stimulated Raman spectroscopy and microscopy. Phys. Rev. Lett. 112, 053905 (2014).
Isobe, K. et al. Simultaneous imaging of two-photon absorption and stimulated Raman scattering by spatial overlap modulation nonlinear optical microscopy. Biomed. Opt. Express 4, 1548–1558 (2013).
Hashimoto, M., Araki, T. & Kawata, S. Molecular vibration imaging in the fingerprint region by use of coherent anti-Stokes Raman scattering microscopy with a collinear configuration. Opt. Lett. 25, 1768–1770 (2000).
Cheng, J-X., Book, L. D. & Xie, X. S. Polarization coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 26, 1341–1343 (2001).
Garbacik, E. T. et al. Background-free nonlinear microspectroscopy with vibrational molecular interferometry. Phys. Rev. Lett. 107, 253902 (2011).
Potma, E. O., Evans, C. L. & Xie, X. S. Heterodyne coherent anti-Stokes Raman scattering (CARS) imaging. Opt. Lett. 31, 241–243 (2006).
Evans, C. L. & Xie, X. S. Coherent anti-stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 1, 883–909 (2008).
Scully, M. O. et al. FAST CARS: engineering a laser spectroscopic technique for rapid identification of bacterial spores. Proc. Natl Acad. Sci. USA 99, 10994–11001 (2002).
Hellerer, T., Enejder, A. M. K. & Zumbusch, A. Spectral focusing: high spectral resolution spectroscopy with broad-bandwidth laser pulses. Appl. Phys. Lett. 85, 25–27 (2004).
Bégin, S. et al. Coherent anti-Stokes Raman scattering hyperspectral tissue imaging with a wavelength-swept system. Biomed. Opt. Express 2, 1296–1306 (2011).
Pegoraro, A. F., Slepkov, A. D., Ridsdale, A., Moffatt, D. J. & Stolow, A. Hyperspectral multimodal CARS microscopy in the fingerprint region. J. Biophoton. 7, 49–58 (2014).
Di Napoli, C. et al. Hyperspectral and differential CARS microscopy for quantitative chemical imaging in human adipocytes. Biomed. Opt. Express 5, 1378–1390 (2014).
Kong, L. et al. Multicolor stimulated Raman scattering microscopy with a rapidly tunable optical parametric oscillator. Opt. Lett. 38, 145–147 (2013).
Ozeki, Y. et al. High-speed molecular spectral imaging of tissue with stimulated Raman scattering. Nature Photon. 6, 845–851 (2012).
Zhang, D. et al. Quantitative vibrational imaging by hyperspectral stimulated Raman scattering microscopy and multivariate curve resolution analysis. Anal. Chem. 85, 98–106 (2013).
Beier, H. T., Noojin, G. D. & Rockwell, B. A. Stimulated Raman scattering using a single femtosecond oscillator with flexibility for imaging and spectral applications. Opt. Express 19, 18885–18892 (2011).
Prince, B. D., Chakraborty, A., Prince, B. M. & Stauffer, H. U. Development of simultaneous frequency- and time-resolved coherent anti-Stokes Raman scattering for ultrafast detection of molecular Raman spectra. J. Chem. Phys. 125, 44502 (2006).
Pestov, D. et al. Optimizing the laser-pulse configuration for coherent Raman spectroscopy. Science 316, 265–268 (2007).
Kano, H. & Hamaguchi, H. Femtosecond coherent anti-Stokes Raman scattering spectroscopy using supercontinuum generated from a photonic crystal fiber. Appl. Phys. Lett. 85, 4298–4300 (2004).
Lee, Y. J. & Cicerone, M. T. Vibrational dephasing time imaging by time-resolved broadband coherent anti-Stokes Raman scattering microscopy. Appl. Phys. Lett. 92, 041108 (2008).
Selm, R. et al. Ultrabroadband background-free coherent anti-Stokes Raman scattering microscopy based on a compact Er:fiber laser system. Opt. Lett. 35, 3282–3284 (2010).
Benalcazar, W. A. et al. High-speed nonlinear interferometric vibrational imaging of biological tissue with comparison to Raman microscopy. IEEE J. Quantum Electron. 16, 824–832 (2009).
Chowdary, P. D. et al. Molecular histopathology by spectrally reconstructed nonlinear interferometric vibrational imaging. Cancer Res. 70, 9562–9569 (2010).
Kano, H. & Hamaguchi, H-O. Vibrationally resonant imaging of a single living cell by supercontinuum-based multiplex coherent anti-Stokes Raman scattering microspectroscopy. Opt. Express 13, 1322–1327 (2005).
Cui, M., Bachler, B. R. & Ogilvie, J. P. Comparing coherent and spontaneous Raman scattering under biological imaging conditions. Opt. Lett. 34, 773–775 (2009).
Vartiainen, E. M. Phase retrieval approach for coherent anti-Stokes Raman scattering spectrum analysis. J. Opt. Soc. Am. B 9, 1209–1214 (1992).
Rinia, H. A., Bonn, M., Vartiainen, E. M., Schaffer, C. B. & Müller, M. Spectroscopic analysis of the oxygenation state of hemoglobin using coherent anti-Stokes Raman scattering. J. Biomed. Opt. 11, 050502 (2006).
Vartiainen, E. M., Rinia, H. A., Müller, M. & Bonn, M. Direct extraction of Raman line-shapes from congested CARS spectra. Opt. Express 14, 3622–3630 (2006).
Rinia, H. A., Bonn, M., Müller, M. & Vartiainen, E. M. Quantitative CARS spectroscopy using the maximum entropy method: the main lipid phase transition. Chemphyschem 8, 279–287 (2007).
Petrov, G. I. et al. Comparison of coherent and spontaneous Raman microspectroscopies for noninvasive detection of single bacterial endospores. Proc. Natl Acad. Sci. USA 104, 7776–7779 (2007).
Rinia, H. A., Burger, K. N. J., Bonn, M. & Müller, M. Quantitative label-free imaging of lipid composition and packing of individual cellular lipid droplets using multiplex CARS microscopy. Biophys. J. 95, 4908–4914 (2008).
Pohling, C., Buckup, T., Pagenstecher, A. & Motzkus, M. Chemoselective imaging of mouse brain tissue via multiplex CARS microscopy. Biomed. Opt. Express 2, 2110–2116 (2011).
Liu, Y., Lee, Y. J. & Cicerone, M. T. Broadband CARS spectral phase retrieval using a time-domain Kramers–Kronig transform. Opt. Lett. 34, 1363–1365 (2009).
Cicerone, M. T., Aamer, K. A., Lee, Y. J. & Vartiainen, E. Maximum entropy and time-domain Kramers–Kronig phase retrieval approaches are functionally equivalent for CARS microspectroscopy. J. Raman Spectrosc. 43, 637–643 (2012).
Lee, Y. J., Moon, D., Migler, K. B. & Cicerone, M. T. Quantitative image analysis of broadband CARS hyperspectral images of polymer blends. Anal. Chem. 83, 2733–2739 (2011).
Parekh, S. H., Lee, Y. J., Aamer, K. A. & Cicerone, M. T. Label-free cellular imaging by broadband coherent anti-Stokes Raman scattering microscopy. Biophys. J. 99, 2695–2704 (2010).
Rinia, H. A., Bonn, M. & Müller, M. Quantitative multiplex CARS spectroscopy in congested spectral regions. J. Phys. Chem. B 110, 4472–4479 (2006).
Arora, R., Petrov, G. I., Yakovlev, V. V. & Scully, M. O. Detecting anthrax in the mail by coherent Raman microspectroscopy. Proc. Natl Acad. Sci. USA 109, 1151–1153 (2012).
von Vacano, B., Meyer, L. & Motzkus, M. Rapid polymer blend imaging with quantitative broadband multiplex CARS microscopy. J. Raman Spectrosc. 38, 916–926 (2007).
Wurpel, G. W. H., Schins, J. M. & Müller, M. Chemical specificity in three-dimensional imaging with multiplex coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 27, 1093–5 (2002).
Jüngst, C., Winterhalder, M. J. & Zumbusch, A. Fast and long term lipid droplet tracking with CARS microscopy. J. Biophoton. 4, 435–441 (2011).
Matthäus, C. et al. In vivo characterization of atherosclerotic plaque depositions by Raman-probe spectroscopy and in vitro coherent anti-stokes Raman scattering microscopic imaging on a rabbit model. Anal. Chem. 84, 7845–7851 (2012).
Kim, S-H. et al. Multiplex coherent anti-stokes Raman spectroscopy images intact atheromatous lesions and concomitantly identifies distinct chemical profiles of atherosclerotic lipids. Circ. Res. 106, 1332–1341 (2010).
Le, T. T., Langohr, I. M., Locker, M. J., Sturek, M. & Cheng, J-X. Label-free molecular imaging of atherosclerotic lesions using multimodal nonlinear optical microscopy. J. Biomed. Opt. 12, 054007 (2007).
Romer, T. J. et al. Histopathology of human coronary atherosclerosis by quantifying its chemical composition with Raman spectroscopy. Circulation 97, 878–885 (1998).
Le, T. T., Huff, T. B. & Cheng, J-X. Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis. BMC Cancer 9, 42 (2009).
Mitra, R., Chao, O., Urasaki, Y., Goodman, O. B. & Le, T. T. Detection of lipid-rich prostate circulating tumour cells with coherent anti-Stokes Raman scattering microscopy. BMC Cancer 12, 540 (2012).
Fu, Y., Wang, H., Huff, T. B., Shi, R. & Cheng, J-X. Coherent anti-Stokes Raman scattering imaging of myelin degradation reveals a calcium-dependent pathway in lyso-PtdCho-induced demyelination. J. Neurosci. Res. 85, 2870–2881 (2007).
Imitola, J. et al. Multimodal coherent anti-Stokes Raman scattering microscopy reveals microglia-associated myelin and axonal dysfunction in multiple sclerosis-like lesions in mice. J. Biomed. Opt. 16, 021109 (2011).
Bélanger, E. et al. In vivo evaluation of demyelination and remyelination in a nerve crush injury model. Biomed. Opt. Express 2, 2698–2708 (2011).
Ji, M. et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci. Transl. Med. 5, 1–10 (2013).
Konorov, S. O. et al. In situ analysis of living embryonic stem cells by coherent anti-stokes Raman microscopy. Anal. Chem. 79, 7221–7225 (2007).
Mouras, R., Bagnaninchi, P. O., Downes, A. R. & Elfick, A. P. D. Label-free assessment of adipose-derived stem cell differentiation using coherent anti-Stokes Raman scattering and multiphoton microscopy. J. Biomed. Opt. 17, 116011 (2012).
Lee, Y. J. et al. Quantitative, label-free characterization of stem cell differentiation at the single-cell level by broadband coherent anti-Stokes Raman scattering microscopy. Tissue Eng. Part C. Methods 20, 562–569 (2014).
De Grauw, C. J., Otto, C. & Greve, J. Line-scan Raman microspectrometry for biological applications. Appl. Spectrosc. 51, 1607–1612 (1997).
Afonso, P. V. et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell 22, 1079–1091 (2012).
Hartshorn, C. M. et al. Multicomponent chemical imaging of pharmaceutical solid dosage forms with broadband CARS microscopy. Anal. Chem. 85, 8102–8111 (2013).
Windbergs, M. et al. Chemical imaging of oral solid dosage forms and changes upon dissolution using coherent anti-Stokes Raman scattering microscopy. Anal. Chem. 81, 2085–2091 (2009).
Matthews, Q., Brolo, A., Lum, J., Duan, X. & Jirasek, A. Raman spectroscopy of single human tumour cells exposed to ionizing radiation in vitro. Phys. Med. Biol. 56, 19–38 (2011).
Chen, B-C., Sung, J. & Lim, S-H. Chemical imaging with frequency modulation coherent anti-Stokes Raman scattering microscopy at the vibrational fingerprint region. J. Phys. Chem. B 114, 16871–16880 (2010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Camp Jr, C., Cicerone, M. Chemically sensitive bioimaging with coherent Raman scattering. Nature Photon 9, 295–305 (2015). https://doi.org/10.1038/nphoton.2015.60
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2015.60
This article is cited by
-
Photoswitchable polyynes for multiplexed stimulated Raman scattering microscopy with reversible light control
Nature Communications (2024)
-
Noise learning of instruments for high-contrast, high-resolution and fast hyperspectral microscopy and nanoscopy
Nature Communications (2024)
-
Upconversion time-stretch infrared spectroscopy
Light: Science & Applications (2023)
-
Fast histological assessment of adipose tissue inflammation by label-free mid-infrared optoacoustic microscopy
npj Imaging (2023)
-
Label-free mid-infrared photothermal live-cell imaging beyond video rate
Light: Science & Applications (2023)