Abstract
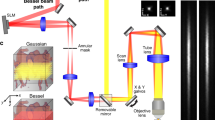
To date, brain imaging has largely relied on X-ray computed tomography and magnetic resonance angiography, with their limited spatial resolution and long scanning times. Fluorescence-based brain imaging in the visible and traditional near-infrared regions (400–900 nm) is an alternative, but at present it requires craniotomy, cranial windows and skull-thinning techniques, and the penetration depth is limited to 1–2 mm due to light scattering. Here, we report through-scalp and through-skull fluorescence imaging of mouse cerebral vasculature without craniotomy, utilizing the intrinsic photoluminescence of single-walled carbon nanotubes in the 1.3–1.4 μm near-infrared window (NIR-IIa window). Reduced photon scattering in this spectral region allows fluorescence imaging to a depth of >2 mm in mouse brain with sub-10-μm resolution. An imaging rate of ∼5.3 frames per second allows for dynamic recording of blood perfusion in the cerebral vessels with sufficient temporal resolution, providing real-time assessment of a blood flow anomaly in a mouse middle cerebral artery occlusion stroke model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Go, A. S. et al. Heart disease and stroke statistics—2013 update—a report from the American Heart Association. Circulation 127, E6–E245 (2013).
Schramm, P. et al. Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke 33, 2426–2432 (2002).
Wright, S. N. et al. Digital reconstruction and morphometric analysis of human brain arterial vasculature from magnetic resonance angiography. Neuroimage 82, 170–181 (2013).
Huang, C.-H. et al. High-resolution structural and functional assessments of cerebral microvasculature using 3D gas ΔR2*-mMRA. PloS ONE 8, e78186 (2013).
Flohr, T. G. et al. First performance evaluation of a dual-source CT (DSCT) system. Eur. Radiol. 16, 256–268 (2006).
Jacoby, C. et al. Dynamic changes in murine vessel geometry assessed by high-resolution magnetic resonance angiography: a 9.4 T study. J. Magn. Reson. Imaging 28, 637–645 (2008).
Paulus, M. J., Gleason, S. S., Kennel, S. J., Hunsicker, P. R. & Johnson, D. K. High resolution X-ray computed tomography: an emerging tool for small animal cancer research. Neoplasia 2, 62–70 (2000).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nature Photon. 7, 205–209 (2013).
Svoboda, K., Denk, W., Kleinfeld, D. & Tank, D. W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997).
Drew, P. J. et al. Chronic optical access through a polished and reinforced thinned skull. Nature Methods 7, 981–984 (2010).
Yang, G., Pan, F., Parkhurst, C. N., Grutzendler, J. & Gan, W.-B. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nature Protoc. 5, 201–208 (2010).
Martirosyan, N. L. et al. Use of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor. Laboratory investigation. J. Neurosurg. 115, 1131–1138 (2011).
Theer, P., Hasan, M. T. & Denk, W. Two-photon imaging to a depth of 1000 µm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 28, 1022–1024 (2003).
Kobat, D., Horton, N. G. & Xu, C. In vivo two-photon microscopy to 1.6-mm depth in mouse cortex. J. Biomed. Opt. 16, 106014 (2011).
Splinter, R. & Hooper, B. A. An Introduction to Biomedical Optics (Taylor & Francis, 2007).
Frangioni, J. V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 7, 626–634 (2003).
Smith, A. M., Mancini, M. C. & Nie, S. Bioimaging: second window for in vivo imaging. Nature Nanotech. 4, 710–711 (2009).
Yi, H. et al. M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors. Nano Lett. 12, 1176–1183 (2012).
Welsher, K. et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotech. 4, 773–780 (2009).
Welsher, K., Sherlock, S. P. & Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl Acad. Sci. USA 108, 8943–8948 (2011).
Hong, G. et al. Three-dimensional imaging of single nanotube molecule endocytosis on plasmonic substrates. Nature Commun. 3, 700 (2012).
Hong, G. et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nature Med. 18, 1841–1846 (2012).
Won, N. et al. Imaging depths of near-infrared quantum dots in first and second optical windows. Mol. Imaging 11, 338–352 (2012).
Hong, G. et al. In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angew. Chem. Int. Ed. 51, 9818–9821 (2012).
Naczynski, D. J. et al. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nature Commun. 4, 2199 (2013).
Tao, Z. et al. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. Int. Ed. 52, 13002–13006 (2013).
Dong, B. et al. Facile synthesis of highly photoluminescent Ag2Se quantum dots as a new fluorescent probe in the second near-infrared window for in vivo imaging. Chem. Mater. 25, 2503–2509 (2013).
Hong, G. et al. Near-infrared II fluorescence for imaging hindlimb vessel regeneration with dynamic tissue perfusion measurement. Circ. Cardiovasc. Imaging 7, 517–525 (2014).
Hong, G. et al. Ultrafast fluorescence imaging in vivo with conjugated polymer fluorophores in the second near-infrared window. Nature Commun. 5, 4206 10.1038/ncomms5206(2014).
Chiang, I. W. et al. Purification and characterization of single-wall carbon nanotubes (SWNTs) obtained from the gas-phase decomposition of CO (HiPco process). J. Phys. Chem. B 105, 8297–8301 (2001).
O'Connell, M. J. et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 297, 593–596 (2002).
Bachilo, S. M. et al. Structure-assigned optical spectra of single-walled carbon nanotubes. Science 298, 2361–2366 (2002).
Van Staveren, H. J., Moes, C. J. M., van Marie, J., Prahl, S. A. & van Gemert, M. J. C. Light scattering in Intralipid-10% in the wavelength range of 400–1100 nm. Appl. Opt. 30, 4507–4514 (1991).
Curcio, J. A. & Petty, C. C. The near infrared absorption spectrum of liquid water. J. Opt. Soc. Am. 41, 302–304 (1951).
Bashkatov, A. N., Genina, E. A., Kochubey, V. I. & Tuchin, V. V. Optical properties of human cranial bone in the spectral range from 800 to 2000 nm. Saratov Fall Meeting 2005: Optical Technologies in Biophysics and Medicine VII 6163, 616310 (2006).
Bashkatov, A. N., Genina, E. A., Kochubey, V. I. & Tuchin, V. V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D 38, 2543–2555 (2005).
White, J. G., Amos, W. B. & Fordham, M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light-microscopy. J. Cell Biol. 105, 41–48 (1987).
Kleinfeld, D., Mitra, P. P., Helmchen, F. & Denk, W. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc. Natl Acad. Sci. USA 95, 15741–15746 (1998).
Shih, A. Y. et al. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J. Cerebr. Blood Flow Metab. 32, 1277–1309 (2012).
Helmchen, F. & Kleinfeld, D. In vivo measurements of blood flow and glial cell function with two-photon laser-scanning microscopy. Methods Enzymol. 444, 231–254 (2008).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nature Methods 2, 932–940 (2005).
Dunn, A. K. Laser speckle contrast imaging of cerebral blood flow. Ann. Biomed. Eng. 40, 367–377 (2012).
Boas, D. A. & Dunn, A. K. Laser speckle contrast imaging in biomedical optics. J. Biomed. Opt. 15, 011109 (2010).
Vakoc, B. J. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nature Med. 15, 1219–1223 (2009).
Senarathna, J., Rege, A., Li, N. & Thakor, N. V. Laser speckle contrast imaging: theory, instrumentation and applications. IEEE Rev. Biomed. Eng. 6, 99–110 (2013).
Hillman, E. M. C. & Moore, A. All-optical anatomical co-registration for molecular imaging of small animals using dynamic contrast. Nature Photon. 1, 526–530 (2007).
Acknowledgements
This study was supported by the National Cancer Institute of the US National Institutes of Health (NIH, grant 5R01CA135109-02, to H.D.), the American Heart Association (Innovative Science Award to C.J.K.), the NIH (grant 1R01NS064517, to C.J.K.) and a William S. Johnson Fellowship (to G.H.). The authors thank J. M. Pauly, D. Wong and T. Zhang for discussions.
Author information
Authors and Affiliations
Contributions
H.D., C.J.K., G.H., S.D. and J.C. conceived and designed the experiments. G.H., S.D., J.C., A.L.A., C.C., B.Z., S.Z. and D.N.A. performed the experiments. G.H., S.D., J.C., A.L.A., C.C., B.Z., S.Z., D.N.A., P.L.H., K.I.A., C.J.K. and H.D. analysed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1934 kb)
Supplementary movie 1
Supplementary movie 1 (AVI 6074 kb)
Supplementary movie 2
Supplementary movie 2 (AVI 4218 kb)
Supplementary movie 3
Supplementary movie 3 (AVI 3848 kb)
Rights and permissions
About this article
Cite this article
Hong, G., Diao, S., Chang, J. et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nature Photon 8, 723–730 (2014). https://doi.org/10.1038/nphoton.2014.166
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2014.166
This article is cited by
-
Oxyhaemoglobin saturation NIR-IIb imaging for assessing cancer metabolism and predicting the response to immunotherapy
Nature Nanotechnology (2024)
-
Near-infrared luminescence high-contrast in vivo biomedical imaging
Nature Reviews Bioengineering (2023)
-
Time gated Fourier transform spectroscopy as a technique for disentangling short- and long-lived luminescence
Communications Materials (2023)
-
Nanomaterial-based contrast agents
Nature Reviews Methods Primers (2023)
-
Direct and quantitative assessments of near-infrared light attenuation and spectroscopic detection depth in biological tissues using surface-enhanced Raman scattering
Med-X (2023)