Abstract
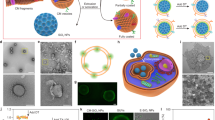
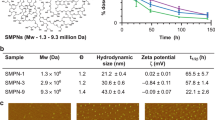
The therapeutic efficacy of systemic drug-delivery vehicles depends on their ability to evade the immune system, cross the biological barriers of the body and localize at target tissues. White blood cells of the immune system—known as leukocytes—possess all of these properties and exert their targeting ability through cellular membrane interactions. Here, we show that nanoporous silicon particles can successfully perform all these actions when they are coated with cellular membranes purified from leukocytes. These hybrid particles, called leukolike vectors, can avoid being cleared by the immune system. Furthermore, they can communicate with endothelial cells through receptor–ligand interactions, and transport and release a payload across an inflamed reconstructed endothelium. Moreover, leukolike vectors retained their functions when injected in vivo, showing enhanced circulation time and improved accumulation in a tumour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bauer, H. C. et al. New aspects of the molecular constituents of tissue barriers. J. Neural Transm. 118, 7–21 (2011).
Rabanel, J. M., Aoun, V., Elkin, I., Mokhtar, M. & Hildgen, P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr. Med. Chem. 19, 3070–3102 (2012).
Owens, D. E. III & Peppas, N. A . Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 307, 93–102 (2006).
Moghimi, S. M. & Davis, S. S. Innovations in avoiding particle clearance from blood by Kupffer cells: cause for reflection. Crit. Rev. Ther. Drug Carrier Syst. 11, 31–59 (1994).
Jain, R. K. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 46, 149–168 (2001).
Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers. Nature Rev. Drug Discov. 4, 145–160 (2005).
Banerjee, S., Li, Y., Wang, Z. & Sarkar, F. H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 269, 226–242 (2008).
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Rev. Drug Discov. 7, 771–782 (2008).
Barreto, J. A. et al. Nanomaterials: applications in cancer imaging and therapy. Adv. Mater. 23, H18–H40 (2011).
Mitragotri, S. & Lahann, J. Physical approaches to biomaterial design. Nature Mater. 8, 15–23 (2009).
Yuan, F. et al. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 54, 3352–3356 (1994).
Yuan, F. et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 55, 3752–3756 (1995).
Champion, J. A., Katare, Y. K. & Mitragotri, S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Rel. 121, 3–9 (2007).
Campbell, R. B. et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 62, 6831–6836 (2002).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Cho, Y. I., Park, S., Jeong, S. Y. & Yoo, H. S. In vivo and in vitro anti-cancer activity of thermo-sensitive and photo-crosslinkable doxorubicin hydrogels composed of chitosan–doxorubicin conjugates. Eur. J. Pharmaceut. Biopharmaceut. 73, 59–65 (2009).
Albrecht, H. Trastuzumab (herceptin(r)): Overcoming resistance in her2-overexpressing breast cancer models. Immunotherapy 2, 795–798 (2010).
Chiappini, C. et al. Tailored porous silicon microparticles: fabrication and properties. ChemPhysChem 11, 1029–1035 (2010).
Godin, B., Tasciotti, E., Liu, X., Serda, R. E. & Ferrari, M. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc. Chem. Res. 44, 979–989 (2011).
Tasciotti, E. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotech. 3, 151–157 (2008).
Ananta, J. S. et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nature Nanotech. 5, 815–821 (2010).
Tanaka, T. et al. Nanotechnology for breast cancer therapy. Biomed. Microdev. 11, 49–63 (2009).
Van de Ven, A. L. et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J. Control. Rel. 158, 148–155 (2012).
Mann, A. P. et al. E-selectin-targeted porous silicon particle for nanoparticle delivery to the bone marrow. Adv. Mater. 23, 278–282 (2011).
Shen, H. et al. Cancer therapy: cooperative, nanoparticle-enabled thermal therapy of breast cancer. Adv. Healthcare Mater. 1, 128–128 (2012).
Michor, F., Liphardt, J., Ferrari, M. & Widom, J. What does physics have to do with cancer? Nature Rev. Cancer 11, 657–670 (2011).
Moghimi, S. M. & Szebeni, J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid. Res. 42, 463–478 (2003).
Immordino, M. L., Dosio, F. & Cattel, L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 1, 297–315 (2006).
Woodle, M. C. & Storm, G. Long Circulating Liposomes: Old Drugs, New Therapeutics (Springer-Verlag and Landes Bioscience, 1998).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Rel. 65, 271–284 (2000).
Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 63, 131–135 (2011).
Yoo, J. W., Irvine, D. J., Discher, D. E. & Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nature Rev. Drug Discov. 10, 521–535 (2011).
Barenholz, Y. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell. Biochem. 37, 167–215 (2004).
Manchester, M. & Singh, P. Virus-based nanoparticles (VNPs): platform technologies for diagnostic imaging. Adv. Drug Deliv. Rev. 58, 1505–1522 (2006).
Ashley, C. E. et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature Mater. 10, 389–397 (2011).
Merkel, T. J. et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl Acad. Sci. USA 108, 586–591 (2011).
Von Maltzahn, G. et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nature Mater. 10, 545–552 (2011).
Van Vliet, S. J., Gringhuis, S. I., Geijtenbeek, T. B. & van Kooyk, Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nature Immunol. 7, 1200–1208 (2006).
Carman, C. V. Mechanisms for transcellular diapedesis: probing and pathfinding by ‘invadosome-like protrusions'. J. Cell Sci. 122, 3025–3035 (2009).
Millan, J. et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nature Cell Biol. 8, 113–123 (2006).
Yang, L. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood 106, 584–592 (2005).
Rebman, B. Tumor immunology. ACP Med. 206, 137–151 (2011).
Serda, R. E. et al. Mitotic trafficking of silicon microparticles. Nanoscale 1, 250–259 (2009).
Barreiro, O. et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 157, 1233–1245 (2002).
Ferrati, S. et al. Intracellular trafficking of silicon particles and logic-embedded vectors. Nanoscale 2, 1512–1520 (2010).
Ferrari, M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 28, 181–188 (2010).
Petros, R. A. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nature Rev. Drug Discov. 9, 615–627 (2010).
Taurin, S., Nehoff, H. & Greish, K. Anticancer nanomedicine and tumor vascular permeability; where is the missing link? J. Control. Rel. 164, 265–275 (2012).
Trickler, W. J. et al. Silver nanoparticle induced blood–brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol. Sci. 118, 160–170 (2010).
Van de Ven, A. L. et al. Integrated intravital microscopy and mathematical modeling to optimize nanotherapeutics delivery to tumors. AIP Adv. 2, 11208 (2012).
Acknowledgements
The authors acknowledge support from the Alliance for NanoHealth Department of Defence Telemedicine & Advanced Technology Research Center (09-W81XWH-10-2-0125), the Defense Advanced Research Projects Agency (W911NF-11-1-0266), the National Institutes of Health (U54CA143837 and U54CA151668), the Department of Defense/Breast Cancer Research Program (W81XWH-09-1-0212) and by The Methodist Hospital Research Institute including Ernest Cockrell Jr. Distinguished Endowed Chair. A.P. was supported by the Bianca Garavaglia Association (Italy). N.Q. was supported by the Ministero Istruzione Universita Ricerca (Italy). J.O.M. was supported by the National Institutes of Health Center for Clinical and Translational Sciences through Clinical and Translational Award TL1 RR024147 from the National Center for Research Resources. M.V.E. was supported by Euroclone S.p.a. The content of this Article is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors also thank M.E. Masserini, M. Agostini, D. Nitti and F. Hussain for their mentoring role, X. Liu for fabrication of the nanoporous silicon particles, K. Dunner Jr for assistance with transmission electron microscopy, K. Cui and The Methodist Hospital Research Institute Advanced Cellular and Tissue Microscope Core Facility for time-lapse live cell microscopy, D. Haviland and The Methodist Hospital Research Institute Flow Cytometry Core Facility and E. De Rosa for data analysis, N. Warier, S. Scaria, G. Adriani, P. Decuzzi, E.V. Zabre and A. Grattoni for technical support and M.G. Landry for graphical assistance.
Author information
Authors and Affiliations
Contributions
A.P. supervised all cellular experiments, interpreted the data and wrote the manuscript. N.Q. developed and optimized the protocols for leukolike vector assembly. A.V. designed and performed all intravital microscopy experiments and analysis and wrote the paper. C.C. manufactured the NPS. M.E. optimized the development of the system. J.O.M. carried out and analysed time-lapse microscopy experiments. B.B. performed confocal microscopy and flow cytometry. S.K. performed the physical and chemical characterization. I.K.Y. performed SEM and assisted with analysis. M.V.E. performed the transwell assays. L.I. optimized in vitro flow systems. M.F. performed the final edits of the manuscript and mentored the authors during the development of the project. E.T. conceived the LLV concept, wrote the paper and was the principal investigator of the major supporting grants.
Corresponding author
Ethics declarations
Competing interests
Commercialization rights on the intellectual property presented in this paper have been acquired by Leonardo Biosystems, from the title holder, the University of Texas Health Science Center in Houston. M.F. is the founding scientist of Leonardo Biosystems, E.T. is the inventor of the technology and hereby both authors disclose potential financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 10444 kb)
Supplementary movie S1
Supplementary movie S1 (MOV 6524 kb)
Supplementary movie S2
Supplementary movie S2 (MOV 4973 kb)
Supplementary movie S3
Supplementary movie S3 (MOV 3706 kb)
Supplementary movie S4
Supplementary movie S4 (MOV 4156 kb)
Supplementary movie S5
Supplementary movie S5 (MOV 4757 kb)
Supplementary movie S6
Supplementary movie S6 (MOV 5098 kb)
Supplementary movie S7
Supplementary movie S7 (MOV 14272 kb)
Supplementary movie S8
Supplementary movie S8 (MOV 14034 kb)
Rights and permissions
About this article
Cite this article
Parodi, A., Quattrocchi, N., van de Ven, A. et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nature Nanotech 8, 61–68 (2013). https://doi.org/10.1038/nnano.2012.212
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2012.212
This article is cited by
-
Age-associated disparity in phagocytic clearance affects the efficacy of cancer nanotherapeutics
Nature Nanotechnology (2024)
-
Bioinspired nanovesicles released from injectable hydrogels facilitate diabetic wound healing by regulating macrophage polarization and endothelial cell dysfunction
Journal of Nanobiotechnology (2023)
-
Nanomedicine in cancer therapy
Signal Transduction and Targeted Therapy (2023)
-
Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment
Nature Communications (2023)
-
Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs
Nature Reviews Materials (2023)