Abstract
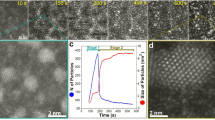
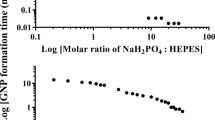
Gold nanoparticles are useful in biomedical applications due to their distinct optical properties and high chemical stability1,2,3,4,5. Reports of the biogenic formation of gold colloids from gold complexes has also led to an increased level of interest in the biomineralization of gold6,7,8,9,10,11,12,13. However, the mechanism responsible for biomolecule-directed gold nanoparticle formation remains unclear due to the lack of structural information about biological systems and the fast kinetics of biomimetic chemical systems in solution. Here we show that intact single crystals of lysozyme can be used to study the time-dependent, protein-directed growth of gold nanoparticles. The protein crystals slow down the growth of the gold nanoparticles, allowing detailed kinetic studies to be carried out, and permit a three-dimensional structural characterization that would be difficult to achieve in solution. Furthermore, we show that additional chemical species can be used to fine-tune the growth rate of the gold nanoparticles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Daniel, M. C. & Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 104, 293–346 (2004).
Rosi, N. L. & Mirkin, C. A. Nanostructures in biodiagnostics. Chem. Rev. 105, 1547–1562 (2005).
De, M. et al. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nature Chem. 1, 461–465 (2009).
Murphy, C. J. et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc. Chem. Res. 41, 1721–1730 (2008).
Xie, J. P., Zheng, Y. G. & Ying, J. Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 131, 888–889 (2009).
Reith, F., Rogers, S. L., McPhail, D. C. & Webb, D. Biomineralization of gold: biofilms on bacterioform gold. Science 313, 233–236 (2006).
Reith, F. et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl Acad. Sci. USA 106, 17757–17762 (2009).
Lengke, M. F. & Southam, G. The effect of thiosulfate-oxidizing bacteria on the stability of the gold–thiosulfate complex. Geochim. Cosmochim. Acta 69, 3759–3772 (2005).
Karthikeyan, S. & Beveridge, T. J. Pseudomonas aeruginosa biofilms react with and precipitate toxic soluble gold. Environ. Microbiol. 4, 667–675 (2002).
Kashefi, K., Tor, J. M., Nevin, K. P. & Lovley, D. R. Reductive precipitation of gold by dissimilatory Fe(III)-reducing bacteria and archaea. Appl. Environ. Microbiol. 67, 3275–3279 (2001).
Karamushka, V. I., Ulberg, Z. R. & Gruzina, T. G. The role of membrane processes in bacterial accumulation of Au(III) and Au(0). Ukr. Biokhimicheskii Zhurnal 62, 76–82 (1990).
Carney, C. K., Harry, S. R., Sewell, S. L. & Wright, D. W. Detoxification biominerals. Top. Curr. Chem. 270, 155–185 (2007).
Mann, S. (eds) Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry (Oxford Univ. Press, 2001).
Meldrum, F. C., Wade, V. J., Nimmo, D. L., Heywood, B. R. & Mann, S. Synthesis of inorganic nanophase materials in supramolecular protein cages. Nature 349, 684–687 (1991).
Uchida, M. et al. Biological containers: protein cages as multifunctional nanoplatforms. Adv. Mater. 19, 1025–1042 (2007).
Medalsy, I. et al. SP1 protein-based nanostructures and arrays. Nano Lett. 8, 473–477 (2008).
Butts, C. A. et al. Directing noble metal ion chemistry within a designed ferritin protein. Biochemistry 47, 12729–12739 (2008).
Ueno, T. et al. Process of accumulation of metal ions on the interior surface of apo-ferritin: crystal structures of a series of apo-ferritins containing variable quantities of Pd(II) Ions. J. Am. Chem. Soc. 131, 5094–5100 (2009).
Ueno, T., Abe, S., Yokoi, N. & Watanabe, Y. Coordination design of artificial metalloproteins utilizing protein vacant space. Coord. Chem. Rev. 251, 2717–2731 (2007).
Dickerson, M. B., Sandhage, K. H. & Naik, R. R. Protein- and peptide-directed syntheses of inorganic materials. Chem. Rev. 108, 4935–4978 (2008).
Margolin, A. L. & Navia, M. A. Protein crystals as novel catalytic materials. Angew. Chem. Int. Ed. 40, 2205–2222 (2001).
Koshiyama, T. et al. Modification of porous protein crystals in development of biohybrid materials. Bioconjugate Chem. 21, 264–269 (2010).
Falkner, J. C. et al. Virus crystals as nanocomposite scaffolds. J. Am. Chem. Soc. 127, 5274–5275 (2005).
Guli, M., Lambert, E. M., Li, M. & Mann, S. Template-directed synthesis of nanoplasmonic arrays by intracrystalline metalization of cross-linked lysozyme crystals. Angew. Chem. Int. Ed. 49, 520–523 (2010).
Jolles, P. & Jolles, J. Whats new in lysozyme research—always a model system, today as yesterday. Mol. Cell. Biochem. 63, 165–189 (1984).
Sanders, L. K. et al. Control of electrostatic interactions between F-actin and genetically modified lysozyme in aqueous media. Proc. Natl Acad. Sci. USA 104, 15994–15999 (2007).
Kawamichi, T., Haneda, T., Kawano, M. & Fujita, M. X-ray observation of a transient hemiaminal trapped in a porous network. Nature 461, 633–635 (2009).
Li, H. Y., Xin, H. L., Muller, D. A. & Estroff, L. A. Visualizing the 3D internal structure of calcite single crystals grown in agarose hydrogels. Science 326, 1244–1247 (2009).
Hodzhaoglu, F., Kurniawan, F., Mirsky, V. & Nanev, C. Gold nanoparticles induce protein crystallization. Cryst. Res. Technol. 43, 588–593 (2008).
Arslan, I., Yates, T. J. V., Browning, N. D. & Midgley, P. A. Embedded nanostructures revealed in three dimensions. Science 309, 2195–2198 (2005).
Browning, N. D., Chisholm, M. F. & Pennycook, S. J. Atomic-resolution chemical analysis using a scanning transmission electron microscope. Nature 366, 143–146 (1993).
Puddephatt, R. J. (eds) The Chemistry of Gold (Topics in Inorganic and General Chemistry, Monograph 16) (Elsevier Scientific Publishing, 1978).
Pyykko, P. Theoretical chemistry of gold. Angew. Chem. Int. Ed. 43, 4412–4456 (2004).
Ralle, M., Lutsenko, S. & Blackburn, N. J. X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines. J. Biol. Chem. 278, 23163–23170 (2003).
Lichtenegger, H. C., Schoberl, T., Bartl, M. H., Waite, H. & Stucky, G. D. High abrasion resistance with sparse mineralization: copper biomineral in worm jaws. Science 298, 389–392 (2002).
Acknowledgements
This work was supported by the US National Science Foundation (CMMI 0749028 and DMR-0117792). The authors thank C. Lei and J. Wen for help with the (S)TEM imaging, L.A. Miller for the preparation of microtome samples and 75 kV TEM imaging, J. Soares for solid-state absorption spectroscopic measurements, M. Sardela for XRD measurements, and Y.-W. Lin, N.M. Marshall, S.-L. Tian, H.E. Ihms and K.-D. Miner for helpful discussions. J.Z. and J.M.Z. are supported by DOE DEFG02-01ER45923. S.H. and I.M.R. acknowledge support from the US Department of Energy (grant DE-FC36-05GO15064). (S)TEM experiments were carried out in part in the Frederick Seitz Materials Research Laboratory Central Facilities, University of Illinois.
Author information
Authors and Affiliations
Contributions
H.W., Z.W. and Y.L. designed the research. H.W., Z.W., J.Z., Y.-G.G., L.Y. and H.R. performed the research. H.W., Z.W., J.Z., S.H., Y.-G.G., L.Y., H.R., L.H.T., H.X., C.H., I.M.R., J.-M.Z. and Y.L. analysed the data. All authors co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wei, H., Wang, Z., Zhang, J. et al. Time-dependent, protein-directed growth of gold nanoparticles within a single crystal of lysozyme. Nature Nanotech 6, 93–97 (2011). https://doi.org/10.1038/nnano.2010.280
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.280
This article is cited by
-
Dentin bond strength and antimicrobial activities of universal adhesives containing silver nanoparticles synthesized with Rosa canina extract
Clinical Oral Investigations (2023)
-
Macromolecular protein crystallisation with biotemplate of live cells
Scientific Reports (2022)
-
Design of a gold clustering site in an engineered apo-ferritin cage
Communications Chemistry (2022)
-
Gold/alpha-lactalbumin nanoprobes for the imaging and treatment of breast cancer
Nature Biomedical Engineering (2020)
-
Ultrafast synthesis of carbon quantum dots from fenugreek seeds using microwave plasma enhanced decomposition: application of C-QDs to grow fluorescent protein crystals
Scientific Reports (2020)