Abstract
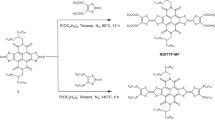
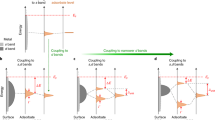
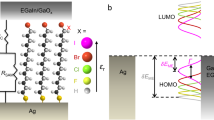
Molecular electronic devices require stable and highly conductive contacts between the metal electrodes and molecules. Thiols and amines are widely used to attach molecules to metals, but they form poor electrical contacts and lack the robustness required for device applications. Here, we demonstrate that dithiocarbamates provide superior electrical contact and thermal stability when compared to thiols on metals. Ultraviolet photoelectron spectroscopy and density functional theory show the presence of electronic states at 0.6 eV below the Fermi level of Au, which effectively reduce the charge injection barrier across the metal−molecule interface. Charge transport measurements across oligophenylene monolayers reveal that the conductance of terphenyl–dithiocarbamate junctions is two orders of magnitude higher than that of terphenyl–thiolate junctions. The stability and low contact resistance of dithiocarbamate-based molecular junctions represent a significant step towards the development of robust, organic-based electronic circuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mann, B. & Kuhn, H. Tunneling through fatty acid salt monolayers. J. Appl. Phys. 42, 4398–4405 (1971).
Aviram, A. & Ratner, M. A. Molecular rectifiers. Chem. Phys. Lett. 29, 277–283 (1974).
Joachim, C., Gimzewski, J. K. & Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 408, 541–548 (2000).
Carroll, R. L. & Gorman, C. B. The genesis of molecular electronics. Angew. Chem. Int. Ed. 41, 4379–4400 (2002).
Akkerman, H. B. & de Boer, B. Electrical conduction through single molecules and self-assembled monolayers. J. Phys. Condens. Matter 20, 013001 (2008).
Tao, N. J. Electron transport in molecular junctions. Nature Nanotech. 1, 173–181 (2006).
Liljeroth, P., Repp, J. & Meyer, G. Current-induced hydrogen tautomerization and conductance switching of naphthalocyanine molecules. Science 317, 1203–1206 (2007).
Nitzan, A. & Ratner, M. A. Electron transport in molecular wire junctions. Science 300, 1384–1389 (2003).
Moth-Poulsen, K. & Bjornholm, T. Molecular electronics with single molecules in solid-state devices. Nature Nanotech. 4, 551–556 (2009).
International Technology Roadmap for Semiconductors (Semiconductor Industry Association, 2009). Available at http://www.itrs.net/Links/2009ITRS/Home2009.htm
Nuzzo, R. G. & Allara, D. L. Adsorption of bifunctional organic disulphides on gold surfaces. J. Am. Chem. Soc. 105, 4481–4483 (1983).
Ulman, A. An Introducton to Ultrathin Organic Films (Academic Press, 1991).
Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 65, 151–256 (2000).
Xue, Y., Datta, S. & Ratner, M. A. Charge transfer and ‘band lineup’ in molecular electronic devices: a chemical and numerical interpretation. J. Chem. Phys. 115, 4292–4299 (2001).
Ishida, T. et al. High resolution X-ray photoelectron spectroscopy measurements of octadecanethiol self-assembled monolayers on Au(111). Langmuir 14, 2092–2096 (1998).
Sellers, H., Ulman, A., Shnidman, Y. & Eilers, J. E. Structure and binding of alkanethiolates on gold and silver surfaces: implications for self-assembled monolayers. J. Am. Chem. Soc. 115, 9389–9401 (1993).
Kornilovitch, P. E. & Bratkovsky, A. M. Orientational dependence of current through molecular films. Phys. Rev. B 64, 195413 (2001).
Donhauser, Z. J. et al. Conductance switching in single molecules through conformational changes. Science 292, 2303–2307 (2001).
Ramachandran, G. K. et al. A bond-fluctuation mechanism for stochastic switching in wired molecules. Science 300, 1413–1416 (2003).
Schreiber, F. et al. Adsorption mechanisms, structures and growth regimes of an archetypal self-assembling system: decanethiol on Au(111). Phys. Rev. B 57, 12476–12481 (1998).
Coucouvanis, D. The chemistry of the dithioacid and 1,1-dithiolate complexes. Prog. Inorg. Chem. 11, 233–371 (1970).
Thorn, G. D. & Ludwig, R. A. The Dithiocarbamates and Related Compounds (Elsevier, 1962).
Wessels, J. M. et al. Optical and electrical properties of three-dimensional interlinked gold nanoparticle assemblies. J. Am. Chem. Soc. 126, 3349–3356 (2004).
Zhao, Y., Pérez-Segarra, W., Shi, Q. & Wei, A. Dithiocarbamate assembly on gold. J. Am. Chem. Soc. 127, 7328–7329 (2005).
Morf, P. et al. Dithiocarbamates: functional and versatile linkers for the formation of self-assembled monolayers. Langmuir 22, 658–663 (2006).
Li, Z. & Kosov, D. S. Dithiocarbamate anchoring in molecular wire junctions: a first principles study. J. Phys. Chem. B 110, 9893–9898 (2006).
von Wrochem, F., Scholz, F., Yasuda, A. & Wessels, J. M. Probing structure and molecular conductance in highly ordered benzyl mercaptan monolayers. J. Phys. Chem. C 113, 12395–12401 (2009).
Azzam, W., Bashir, A., Terfort, A., Strunskus, T. & Woll, C. Combined STM and FTIR characterization of terphenylalkanethiol monolayers on Au(111): effect of alkyl chain length and deposition temperature. Langmuir 22, 3647–3655 (2006).
Laibinis, P. E. et al. Comparison of the structures and wetting properties of self-assembled monolayers of n-alkanethiols on the coinage metal surfaces, Cu, Ag, Au. J. Am. Chem. Soc. 113, 7152–7167 (1991).
Naumann, R. et al. Tethered lipid bilayers on ultraflat gold surfaces. Langmuir 19, 5435–5443 (2003).
Holmlin, R. E. et al. Electron transport through thin organic films in metal−insulator−metal junctions based on self-assembled monolayers. J. Am. Chem. Soc. 123, 5075–5085 (2001).
Weiss, E. A. et al. Influence of defects on the electrical characteristics of mercury-drop junctions: self-assembled monolayers of n-alkanethiolates on rough and smooth silver. J. Am. Chem. Soc. 129, 4336–4349 (2007).
Beebe, J. M., Kim, B., Frisbie, C. D. & Kushmerick, J. G. Measuring relative barrier heights in molecular electronic junctions with transition voltage spectroscopy. ACS Nano 2, 827–832 (2008).
Duwez, A.-S. et al. Surface molecular structure of self-assembled alkanethiols evidenced by UPS and photoemission with synchrotron radiation. J. Phys. Chem. B 101, 884–890 (1997).
Gokhale, S. et al. Electronic structure of benzene adsorbed on single-domain Si(001)–(2x1): a combined experimental and theoretical study. J. Chem. Phys. 108, 5554–5564 (1998).
Campbell, I. H. et al. Controlling Schottky energy barriers in organic electronic devices using self-assembled monolayers. Phys. Rev. B 54, 14321–14324 (1996).
Alloway, D. M. et al. Interface dipoles arising from self-assembled monolayers on gold: UV-photoemission studies of alkanethiols and partially fluorinated alkanethiols. J. Phys. Chem. B 107, 11690–11699 (2003).
Patrone, L. et al. Evidence of the key role of metal−molecule bonding in metal−molecule−metal transport experiments. Phys. Rev. Lett. 91, 096802 (2003).
Di Felice, R., Selloni, A. & Molinari, E. DFT study of cysteine adsorption on Au(111). J. Phys. Chem. B 107, 1151–1156 (2003).
Monachesi, P., Chiodo, L. & Del Sole, R. Ab initio characterization of surface states at the S/Cu(100) interface. Phys. Rev. B 69, 165404 (2004).
Di Ventra, M., Pantelides, S. T. & Lang, N. D. First-principles calculation of transport properties of a molecular device. Phys. Rev. Lett. 84, 979–982 (2000).
Mujica, V. & Ratner, M. A. Current−voltage characteristics of tunneling molecular junctions for off-resonance injection. Chem. Phys. 264, 365–370 (2001).
Grave, C. et al. Charge transport through oligoarylene self-assembled monolayers: interplay of molecular organization, metal−molecule interactions and electronic structure. Adv. Func. Mater. 17, 3816–3828 (2007).
Redhead, P. A. Thermal desorption of gases. Vacuum 12, 203–211 (1962).
Love, J. C., Estroff, L. A., Kriebel, J. K., Nuzzo, R. G. & Whitesides, G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105, 1103–1169 (2005).
Hammer, B., Hansen, L. B. & Norskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Segall, M. D. et al. First-principles simulation: ideas, illustrations and the CASTEP code. J. Phys. Condens. Matt. 14, 2717–2744 (2002).
Agron, P. A. & Carlson, T. A. Angular resolved UPS and XPS spectra of benzenethiol adsorbed on Cu(111) at 300 °K. J. Vac. Sci. Technol. 20, 815–817 (1982).
Acknowledgements
The authors thank Y. Joseph, W.E. Ford, C. Schönenberger, M. Rampi, A. Baratoff, P. Morf and T. Jung for helpful discussions. They also thank C. Menke from Accelrys for support regarding molecular modelling.
Author information
Authors and Affiliations
Contributions
F.v.W. and J.M.W. designed the experiments and coordinated the project. D.G. synthesized the oligophenylenes. H.-G.N. supported the synthesis procedures. F.S. and F.v.W. performed the experiments and analysed the data. F.v.W. carried out the simulations and wrote the paper. G.N. is the laboratory manager.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1234 kb)
Rights and permissions
About this article
Cite this article
von Wrochem, F., Gao, D., Scholz, F. et al. Efficient electronic coupling and improved stability with dithiocarbamate-based molecular junctions. Nature Nanotech 5, 618–624 (2010). https://doi.org/10.1038/nnano.2010.119
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.119
This article is cited by
-
Concepts in the design and engineering of single-molecule electronic devices
Nature Reviews Physics (2019)
-
Visual Identification of Light-Driven Breakage of the Silver-Dithiocarbamate Bond by Single Plasmonic Nanoprobes
Scientific Reports (2015)
-
Wiring molecules into circuits
Nature Nanotechnology (2013)