Abstract
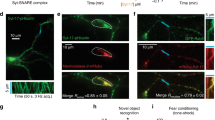
Axons are defined by the presence of presynaptic specializations at specific locations. We show here that loss-of-function mutations in the C. elegans gene syd-1 cause presynaptic specializations to form in the dendritic processes of GABA-expressing motor neurons during initial differentiation. At a later developmental stage, however, syd-1 is not required for the polarity respecification of a subset of these neurons. The SYD-1 protein contains PDZ, C2 and rho–GTPase activating protein (GAP)-like domains, and is localized to presynaptic terminals in mature neurons. A truncated SYD-1 that lacks the rhoGAP domain interferes with neurite outgrowth and guidance. Our data indicate that syd-1 may be involved in specifying axon identity during initial polarity acquisition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Bradke, F. & Dotti, C.G. Changes in membrane trafficking and actin dynamics during axon formation in cultured hippocampal neurons. Microsc. Res. Tech. 48, 3–11 (2000).
Bradke, F. & Dotti, C.G. The role of local actin instability in axon formation. Science 283, 1931–1934 (1999).
Bradke, F. & Dotti, C.G. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19, 1175–1186 (1997).
Andersen, S.S. & Bi, G.Q. Axon formation: a molecular model for the generation of neuronal polarity. Bioessays 22, 172–179 (2000).
McFarlane, S. Dendritic morphogenesis: building an arbor. Mol. Neurobiol. 22, 1–9 (2000).
Luo, L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173–180 (2000).
White, J.G., Southgate, E., Thomson, J.N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B Biol. Sci. 314, 1–340 (1986).
Jin, Y., Jorgensen, E., Hartwieg, E. & Horvitz, H.R. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19, 539–548 (1999).
McIntire, S.L., Jorgensen, E., Kaplan, J. & Horvitz, H.R. The GABAergic nervous system of Caenorhabditis elegans. Nature 364, 337–341 (1993).
Sulston, J.E. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B Biol. Sci. 275, 287–297 (1976).
Sulston, J.E. & Horvitz, H.R. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 56, 110–156 (1977).
Crump, J.G., Zhen, M., Jin, Y. & Bargmann, C.I. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron 29, 115–129 (2001).
Zhen, M. & Jin, Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature 401, 371–375 (1999).
Nonet, M.L. Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. J. Neurosci. Methods 89, 33–40 (1999).
Hallam, S.J. & Jin, Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature 395, 78–82 (1998).
Bamber, B.A., Beg, A.A., Twyman, R.E. & Jorgensen, E.M. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J. Neurosci. 19, 5348–5359 (1999).
Eastman, C., Horvitz, H.R. & Jin, Y. Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. J. Neurosci. 19, 6225–6234 (1999).
Simske, J.S., Kaech, S.M., Harp, S.A. & Kim, S.K. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell 85, 195–204 (1996).
Rongo, C. & Kaplan, J.M. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature 402, 195–199 (1999).
White, J.G., Albertson, D.G. & Anness, M.A. Connectivity changes in a class of motoneurone during the development of a nematode. Nature 271, 764–766 (1978).
Horvitz, H.R. & Sulston, J.E. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics 96, 435–454 (1980).
Rongo, C., Whitfield, C.W., Rodal, A., Kim, S.K. & Kaplan, J.M. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell 94, 751–759 (1998).
Vaduva, G., Martin, N.C. & Hopper, A.K. Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton. J. Cell Biol. 139, 1821–1833 (1997).
Mandai, K. et al. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 139, 517–528 (1997).
Rittinger, K. et al. Crystal structure of a small G protein in complex with the GTPase- activating protein rhoGAP. Nature 388, 693–697 (1997).
Nonet, M.L., Grundahl, K., Meyer, B.J. & Rand, J.B. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell 73, 1291–1305 (1993).
Koushika, S.P. et al. A post-docking role for active zone protein Rim. Nat. Neurosci. 4, 997–1005 (2001).
Hall, D.H. & Hedgecock, E.M. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65, 837–847 (1991).
Ahmari, S.E., Buchanan, J. & Smith, S.J. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat. Neurosci. 3, 445–451 (2000).
Zhai, R.G. et al. Assembling the presynaptic active zone: a characterization of an active zone precursor vesicle. Neuron 29, 131–143 (2001).
Kaether, C., Skehel, P. & Dotti, C.G. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell 11, 1213–1224 (2000).
Wu, W.J., Erickson, J.W., Lin, R. & Cerione, R.A. The γ-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature 405, 800–804 (2000).
McCallum, S.J., Erickson, J.W. & Cerione, R.A. Characterization of the association of the actin-binding protein, IQGAP, and activated Cdc42 with Golgi membranes. J. Biol. Chem. 273, 22537–22544 (1998).
Rittinger, K., Taylor, W.R., Smerdon, S.J. & Gamblin, S.J. Support for shared ancestry of GAPs. Nature 392, 448–449 (1998).
Seewald, M.J., Korner, C., Wittinghofer, A. & Vetter, I.R. RanGAP mediates GTP hydrolysis without an arginine finger. Nature 415, 662–666 (2002).
Craven, S.E. & Bredt, D.S. PDZ proteins organize synaptic signaling pathways. Cell 93, 495–498 (1998).
Dong, H. et al. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386, 279–284 (1997).
Hung, T.J. & Kemphues, K.J. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 126, 127–135 (1999).
Bilder, D. & Perrimon, N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676–680 (2000).
Kay, B.K., Williamson, M.P. & Sudol, M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 14, 231–241 (2000).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Zoller, M. & Smith, M. Oligonucleotide-directed mutatagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. Methods Enzymol. 154, 329–350 (1987).
Clark, S.G., Lu, X. & Horvitz, H.R. The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics 137, 987–997 (1994).
Mello, C.C., Kramer, J.M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Finney, M. & Ruvkun, G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 63, 895–905 (1990).
Acknowledgements
We thank M. Nonet, C. Bargmann, J. Kaplan, B. Herman, B. Bamber, Y. Kohara, A. Coulson and the C. elegans genome consortium for reagents; W. Harris and K. Guan for biochemistry of SYD-1 GAP; B. Ackley, C. Suh, K. Hudson, K. Hoogner, M. Verado and M. Crookham for assistance; A. Chisholm, M. Zhen and our lab members for discussions and comments. We obtained some of these strains from the Caenorhabditis Genetics Center, which is supported by a grant from the National Institutes of Health. This work was funded by a National Science Foundation Presidential Early Career award (Y.J.) and an NSF equipment grant DBI-9729596. S.H. was supported by a GAANN predoctoral fellowship; R.B. was supported by a National Research Service Award postdoctoral fellowship. A.G. is an HHMI research associate, and Y.J. is an assistant investigator of HHMI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1.
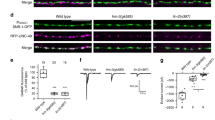
Effect of syd-1 on neuronal morphology, dendro-soma markers and ASI neuron polarity. (a, b) The overall axon morphology and cell body positions of DD and VD motor neurons in wild-type (a) and syd-1 mutant (b). No differences seen. (c, d) The expression of OSM-6::GFP (mnIs17) in phasmid neurons in wild type (c) and syd-1 mutants (d) is the same. Arrowhead, cell bodies; thin arrows, dendrites. (e, f) The expression of GLR-1::GFP (nuIs25) in the ventral cord (arrows) is the same in wild-type (e) and syd-1 mutants (f). (g) In wild-type animals, ASI neurons project a single anterior directed dendrite and a single axonal process fasiculated in the nerve ring, and the expression of SNB::GFP is restricted to the axonal projection (arrow), as illustrated in (i). (h) In syd-1 mutants, fewer SNB::GFP puncta in the axonal region of ASI (arrow) are present and irregular in size, and also mis-localized to the dendritic region (arrowhead), as illustrated in (j). * indicates posteriorly wandered axon. (JPG 41 kb)
Supplementary Fig. 2.
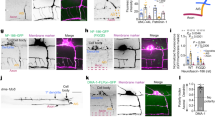
Sequence alignment of PDZ and C2 domains. (a) Alignment of the PDZ domain (residue 55-142). * indicates residues of PDZ-3 of PSD-95 that are required for substrate binding. (b) Alignment of the C2 domain (residue 548-671). * marks the W598A mutation. (GIF 33 kb)
Supplementary Table 1.
Quantitation of cell autonomy for SYD-1 and the effect of mutations in the C2 and GAP domains. Multiple independent transgenic lines for each construct were inspected. Quantitations were made on the staged worms from the most representative lines. (GIF 30 kb)
Rights and permissions
About this article
Cite this article
Hallam, S., Goncharov, A., McEwen, J. et al. SYD-1, a presynaptic protein with PDZ, C2 and rhoGAP-like domains, specifies axon identity in C. elegans. Nat Neurosci 5, 1137–1146 (2002). https://doi.org/10.1038/nn959
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn959
This article is cited by
-
Function of SYDE C2-RhoGAP family as signaling hubs for neuronal development deduced by computational analysis
Scientific Reports (2022)
-
Synapse development and maturation at the drosophila neuromuscular junction
Neural Development (2020)
-
Rapid active zone remodeling consolidates presynaptic potentiation
Nature Communications (2019)
-
Intrinsic and extrinsic mechanisms of synapse formation and specificity in C. elegans
Cellular and Molecular Life Sciences (2019)
-
Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield
Molecular Psychiatry (2017)