Abstract
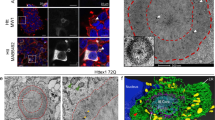
Huntington's disease (HD) is caused by an expansion of exonic CAG triplet repeats in the gene encoding huntingtin protein (Htt), but the mechanisms by which this mutant protein causes neurodegeneration remain unknown. Here we show that lymphoblast mitochondria from patients with HD have a lower membrane potential and depolarize at lower calcium loads than do mitochondria from controls. We found a similar defect in brain mitochondria from transgenic mice expressing full-length mutant huntingtin, and this defect preceded the onset of pathological or behavioral abnormalities by months. By electron microscopy, we identified N-terminal mutant huntingtin on neuronal mitochondrial membranes, and by incubating normal mitochondria with a fusion protein containing an abnormally long polyglutamine repeat, we reproduced the mitochondrial calcium defect seen in human patients and transgenic animals. Thus, mitochondrial calcium abnormalities occur early in HD pathogenesis and may be a direct effect of mutant huntingtin on the organelle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Orr, H.T. Beyond the Qs in the polyglutamine diseases. Genes Dev. 15, 925–932 (2001).
Rigamonti, D. et al. Wild-type huntingtin protects from apoptosis upstream of caspase-3. J. Neurosci. 20, 3705–3713 (2000).
Leavitt, B.R. et al. Wild-type huntingtin reduces the cellular toxicity of mutant huntingtin in vivo. Am. J. Hum. Genet. 68, 313–324 (2001).
Zuccato, C. et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science 293, 493–498 (2001).
Cattaneo, E. et al. Loss of normal huntingtin function: new developments in Huntington's disease research. Trends Neurosci. 24, 182–188 (2001).
Scherzinger, E. et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90, 549–558 (1997).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Davies, S.W. et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90, 537–548 (1997).
Faber, P.W. et al. Huntingtin interacts with a family of WW domain proteins. Hum. Mol. Genet. 7, 1463–1474 (1998).
Wanker, E.E. et al. HIP-I: a huntingtin interacting protein isolated by the yeast two- hybrid system. Hum. Mol. Genet. 6, 487–495 (1997).
Li, X.J. et al. A huntingtin-associated protein enriched in brain with implications for pathology. Nature 378, 398–402 (1995).
Cha, J.H. et al. Altered brain neurotransmitter receptors in transgenic mice expressing a portion of an abnormal human Huntington disease gene. Proc. Natl. Acad. Sci. USA 95, 6480–6485 (1998).
Luthi-Carter, R. et al. Decreased expression of striatal signaling genes in a mouse model of Huntington's disease. Hum. Mol. Genet. 9, 1259–1271 (2000).
Steffan, J.S. et al. The Huntington's disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl. Acad. Sci USA 97, 6763–6768 (2000).
Nucifora, F.C., Jr. et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291, 2423–2428 (2001).
Steffan, J.S. et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743 (2001).
Tabrizi, S.J. et al. Biochemical abnormalities and excitotoxicity in Huntington's disease brain. Ann. Neurol. 45, 25–32 (1999).
Beal, M.F., Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 38, 357–366 (1995).
Greene, J.G. & Greenamyre, J.T. Bioenergetics and glutamate excitotoxicity. Prog. Neurobiol. 48, 613–634 (1996).
Sawa, A. et al. Increased apoptosis of Huntington disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med. 5, 1194–1198 (1999).
Gutekunst, C.A. et al. Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc. Natl. Acad. Sci. USA 92, 8710–8714 (1995).
Hodgson, J.G. et al. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity and selective striatal neurodegeneration. Neuron 23, 181–192 (1999).
Bernardi, P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79, 1127–1155 (1999).
Gutekunst, C.A. et al. The cellular and subcellular localization of huntingtin-associated protein 1 (HAP1): comparison with huntingtin in rat and human. J. Neurosci. 18, 7674–7686 (1998).
Gutekunst, C.A. et al. Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J. Neurosci. 19, 2522–2534 (1999).
Monoi, H., Futaki, S., Kugimiya, S., Minakata, H. & Yoshihara, K. Poly-L-glutamine forms cation channels: relevance to the pathogenesis of the polyglutamine diseases. Biophys. J. 78, 2892–2899 (2000).
Ordway, J.M. et al. Ectopically expressed CAG repeats cause intranuclear inclusions and a progressive late onset neurological phenotype in the mouse. Cell 91, 753–763 (1997).
Zeron, M.M. et al. Increased sensitivity to N-methyl-d-aspartate receptor–mediated excitotoxicity in a mouse model of Huntington's disease. Neuron 33, 849–860 (2002).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306 (2000).
Kim, Y.J. et al. Caspase 3-cleaved N-terminal fragments of wild-type and mutant huntingtin are present in normal and Huntington's disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc. Natl. Acad. Sci. USA 98, 12784–12789 (2001).
Trounce, I.A., Kim, Y.L., Jun, A.S. & Wallace, D.C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 264, 484–509 (1996).
Sims, N.R. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J. Neurochem. 55, 698–707 (1990).
Onodera, O. et al. Oligomerization of expanded-polyglutamine domain fluorescent fusion proteins in cultured mammalian cells. Biochem. Biophys. Res. Commun. 238, 599–605 (1997).
Acknowledgements
This work was supported by the Huntington's Disease Society of America's Coalition for the Cure program (A.V.P., C.A.G., B.R.L., M.R.H. and J.T.G.), the Hereditary Disease Foundation (M.R.H.), the Huntington Society of Canada (M.R.H. and B.R.L.), a Canada Research Chair (M.R.H.), the Canadian Institutes of Health Research (M.R.H. and B.R.L.) and a National Institutes of Health grant AG14648 (J.T.G. and A.V.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Panov, A., Gutekunst, CA., Leavitt, B. et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci 5, 731–736 (2002). https://doi.org/10.1038/nn884
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn884
This article is cited by
-
Neuropathogenesis-on-chips for neurodegenerative diseases
Nature Communications (2024)
-
The updated development of blood-based biomarkers for Huntington’s disease
Journal of Neurology (2023)
-
Soluble mutant huntingtin drives early human pathogenesis in Huntington’s disease
Cellular and Molecular Life Sciences (2023)
-
Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration
Translational Neurodegeneration (2022)
-
Metabolism in Huntington’s disease: a major contributor to pathology
Metabolic Brain Disease (2022)