Abstract
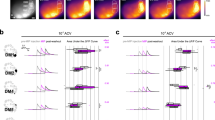
At the first stage of olfactory processing in the brain, synchronous firing across glomeruli may help to temporally bind multiple and spatially distributed input streams activated by a given odor. This hypothesis, however, has never been tested in an organism in which the odor-tuning properties of several spatially identifiable glomeruli are known. Using the sphinx moth, an insect that meets these specific criteria, we recorded odor-evoked responses simultaneously from pairs of projection neurons (PNs) innervating the same or different glomeruli in the macroglomerular complex (MGC), which is involved in processing pheromonal information. PNs that branched in the same glomerulus and were activated by the same pheromone component also showed the strongest coincident responses to each odor pulse. Glomerulus-specific PN pairs were also inhibited by the pheromone component that selectively activated PNs in the neighboring glomerulus, and about 70% of all intraglomerular pairs showed increased synchronization when stimulated with a mixture of the two odorants. Thus, when two adjacent glomeruli receive their inputs simultaneously, the temporal tuning of output from each glomerulus is enhanced by reciprocal and inhibitory interglomerular interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vaadia, E., Ahissar, E., Bergman, H. & Lavner, Y. Correlated activity of neurons: a neural code for higher brain functions? in Neuronal Cooperativity (ed. Kruger, J.) 205–279 (Springer-Verlag, Berlin, 1991).
Rieke, F., Warland, D., de Ruyter van Steveninck, R. & Bialek, W. Spikes: exploring the neural code (MIT Press, Cambridge, Massachusetts, 1997).
Murthy, V. N. & Fetz, E. E. Synchronization of neurons during local field potential oscillations in sensorymotor cortex of awake monkeys. J. Neurophysiol. 76, 3968–3982 (1996).
Singer, W. & Gray, C. M. Visual feature integration and the temporal correlation hypotheses. Annu. Rev. Neurosci. 18, 555–586 (1995).
Adrian, E. D. Olfactory reactions in the brain of the hedgehog. J. Physiol. (Lond.) 100, 459–473 (1942).
Gelperin, A. Oscillatory dynamics and information processing in olfactory systems. J. Exp. Biol. 202, 1855–1864 (1999).
Laurent, G., Wehr, M. & Davidowitz, H. Temporal representations of odors in an olfactory network. J. Neurosci. 16, 3837–3847 (1996).
Mellon, D. & Wheeler, C. J. Coherent oscillations in membrane potential synchronize impulse bursts in central olfactory neurons of the crayfish. J. Neurophysiol. 81, 1231–1241 (1999).
Kashiwadani, H., Sasaki, Y. F., Uchida, N. & Mori, K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J. Neurophysiol. 82, 1786–1792 (1999).
Kay, L. M. & Laurent, G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat. Neurosci. 2, 1003–1009 (1999).
Christensen, T. A., Pawlowski, V. M., Lei, H. & Hildebrand, J. G. Multi-unit recordings reveal context-dependent modulation of synchrony in odor-specific neural ensembles. Nat. Neurosci. 3, 927–931 (2000).
Vickers, N. J., Christensen, T. A., Baker, T. C. & Hildebrand, J. G. Odour-plume dynamics influence the brain's olfactory code. Nature 410, 466–470 (2001).
Christensen, T. A. & White, J. Representation of olfactory information in the brain. in The Neurobiology of Taste and Smell, Vol. 2 (eds. Finger, T. E., Silver, W. L. & Restrepo, D.) 201–232 (Wiley, New York, 2000).
Ressler, K. J., Sullivan, S. L. & Buck, L. B. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell 79, 1245–1255 (1994).
Vassar, R. et al. Topographic organization of sensory projections to the olfactory bulb. Cell 74, 309–318 (1994).
Lancet, D. Vertebrate olfactory reception. Ann. Rev. Neurosci. 9, 329–355 (1986).
Singer, M. S. & Shepherd, G. M. Molecular modeling of ligand-receptor interactions in the OR5 olfactory receptor. Neuroreport 5, 1297–1300 (1994).
Mori, K., Nagao, H. & Yoshihara, Y. The olfactory bulb: coding and processing of odor molecule information. Science 286, 711–715 (1999).
Galizia, C. G. & Menzel, R. The role of glomeruli in the neural representation of odours: results from optical recording studies. J. Insect Physiol. 47, 115–130 (2001).
Shipley, M. T. & Ennis, M. Functional organization of olfactory system. J. Neurobiol. 30, 123–176 (1996).
Stopfer, M., Bhagavan, S., Smith, B. & Laurent, G. Impaired odor discrimination on desynchronization of odour-encoding neural assemblies. Nature 390, 70–74 (1997).
Christensen, T. A., Waldrop, B. R. & Hildebrand, J. G. Multitasking in the olfactory system: context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons. J. Neurosci. 18, 5999–6008 (1998).
Rall, W., Shepherd, G. M., Reese, T. S. & Brightman, M. W. Dendrodentritic synaptic pathway for inhibition in the olfactory bulb. J. Exp. Neurol. 14, 44–56 (1966).
Buonviso, N. & Chaput, M. A. Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single-unit recordings. J. Neurophysiol. 63, 447–454 (1990).
Shepherd, G. M. & Greer, C. A. Olfactory bulb. in The Synaptic Organization of the Brain (ed. Shepherd, G.M.) 139–169 (Oxford, New York, 1990).
Desmaisons, D., Vincent, J. D. & Lledo, P. M. Control of action potential timing by intrinsic subthreshold oscillations in olfactory bulb output neurons. J. Neurosci. 19, 10727–10737 (1999).
Laurent, G. A systems perspective on early olfactory coding. Science 286, 723–728 (1999).
Hansson, B. S., Christensen, T. A. & Hildebrand, J. G. Functionally distinct subdivisions of the macroglomerular complex in the antennal lobes of the sphinx moth Manduca sexta. J. Comp. Neurol. 312, 264–278 (1991).
Heinbockel, T., Christensen, T. A. & Hildebrand, J. G. Temporal tuning of odor responses in pheromone-responsive projection neurons in the brain of the sphinx moth Manduca sexta. J. Comp. Neurol. 409, 1–12 (1999).
Waldrop, B., Christensen, T. A. & Hildebrand, J. G. GABA-mediated synaptic inhibition of projection neurons in the antennal lobes of the sphinx moth Manduca sexta. J. Comp. Physiol. A 161, 23–32 (1987).
Christensen, T. A. & Hildebrand, J. G. Coincident stimulation with pheromone components improves temporal pattern resolution in central olfactory neurons. J. Neurophysiol. 77, 775–781 (1997).
Johnson, B. A., Woo, C. C. & Leon, M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J. Comp. Neurol. 393, 457–471 (1998).
Belluscio, L. & Katz, L. C. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J. Neurosci. 21, 2113–2122 (2001).
Fuss, S. H. & Korsching, S. I. Odorant feature detection: activity mapping of structure response relationships in the zebrafish olfactory bulb. J. Neurosci. 21, 8396–8407 (2001).
Murlis, J., Elkinton, J. S. & Cardé, R. T. Odor plumes and how insects use them. Annu. Rev. Entomol. 37, 505–532 (1992).
Deadwyler, S. A., Bunn, T. & Hampson, R. E. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J. Neurosci. 16, 354–372 (1996).
Nicolelis, M. A. et al. Simultaneous encoding of tactile information by three primate cortical areas. Nat. Neurosci. 1, 621–630 (1998).
Maynard, E. M. et al. Neuronal interactions improve cortical population coding of movement direction. J. Neurosci. 19, 8083–8093 (1999).
Schoppa, N. E. & Westbrook, G. L. Glomerulus-specific synchronization of mitral cells in the olfactory bulb. Neuron 31, 639–651 (2001).
Carlson, G. C., Shipley, M. T. & Keller, A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J. Neurosci. 20, 2011–2021 (2000).
Christensen, T. A. Anatomical and physiological diversity in the central processing of sex-pheromonal information in different moth species. in Insect Pheromone Research: New Directions (eds. Cardé, R.T. & Minks, A.K.) 184–193 (Chapman & Hall, New York, 1997).
Lei, H., Anton, S. & Hansson, B. S. Olfactory protocerebral pathways processing sex pheromone and plant odor information in the male moth Agrotis segetum. J. Comp. Neurol. 432, 356–370 (2001).
Christensen, T. A., Waldrop, B. R., Harrow, I. D. & Hildebrand, J. G. Local interneurons and information processing in the olfactory glomeruli of the moth Manduca sexta. J. Comp. Physiol. A 173, 385–399 (1993).
Rospars, J. P. & Hildebrand, J. G. Anatomical identification of glomeruli in the antennal lobes of the male sphinx moth Manduca sexta. Cell Tissue Res. 270, 205–227 (1992).
Rospars, J. P. & Hildebrand, J. G. Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem. Senses 25, 119–129 (2000).
King, J. R., Christensen, T. A. & Hildebrand, J. G. Response characteristics of an identified, sexually dimorphic olfactory glomerulus. J. Neurosci. 20, 2391–2399 (2000).
Shields, V. D. C. & Hildebrand, J. G. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J. Comp. Physiol. 186, 1135–1151 (2001).
Barlow, H. B., Hill, R. M. & Levick, W. R. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J. Physiol. (Lond.) 173, 377–407 (1964).
Nowak, L. G., Munk, M. H. J., James, A. C., Girard, P. & Bullier, J. Cross-correlation study of the temporal interactions between areas V1 and V2 of the macaque monkey. J. Neurophysiol. 81, 1057–1074 (1999).
Aertsen, A. M. H. J., Gerstein, G. L., Habib, M. K. & Palm, G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J. Neurophysiol. 61, 900–917 (1989).
Acknowledgements
We thank K. Daly, V. Pawlowski and B. Smith for discussions and comments and H. Stein and A.A. Osman for technical assistance. Supported by grants and contracts from National Institutes of Health (NIDCD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lei, H., Christensen, T. & Hildebrand, J. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat Neurosci 5, 557–565 (2002). https://doi.org/10.1038/nn0602-859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn0602-859
This article is cited by
-
Parallel processing in the honeybee olfactory pathway: structure, function, and evolution
Journal of Comparative Physiology A (2013)
-
Glomerular interactions in olfactory processing channels of the antennal lobes
Journal of Comparative Physiology A (2013)
-
Mixture and odorant processing in the olfactory systems of insects: a comparative perspective
Journal of Comparative Physiology A (2013)
-
Synchronous firing of antennal-lobe projection neurons encodes the behaviorally effective ratio of sex-pheromone components in male Manduca sexta
Journal of Comparative Physiology A (2013)
-
Responses of protocerebral neurons in Manduca sexta to sex-pheromone mixtures
Journal of Comparative Physiology A (2013)