Abstract
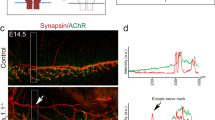
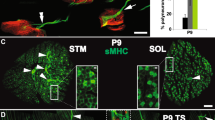
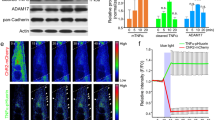
Synapse formation requires proper interaction between pre- and postsynaptic cells. In anterograde signaling, neurons release factors to guide postsynaptic differentiation. However, less is known about how postsynaptic targets retrogradely regulate presynaptic differentiation or function. We found that muscle-specific conditional knockout of β-catenin (Ctnnb1, also known as β-cat) in mice caused both morphologic and functional defects in motoneuron terminals of neuromuscular junctions (NMJs). In the absence of muscle β-catenin, acetylcholine receptor clusters were increased in size and distributed throughout a wider region. Primary nerve branches were mislocated, whereas secondary or intramuscular nerve branches were elongated and reduced in number. Both spontaneous and evoked neurotransmitter release was reduced at the mutant NMJs. Furthermore, short-term plasticity and calcium sensitivity of neurotransmitter release were compromised in β-catenin–deficient muscle. In contrast, the NMJ was normal in morphology and function in motoneuron-specific β-catenin–deficient mice. Taken together, these observations indicate a role for muscle β-catenin in presynaptic differentiation and function, identifying a previously unknown retrograde signaling in the synapse formation and synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kalinovsky, A. & Scheiffele, P. Transcriptional control of synaptic differentiation by retrograde signals. Curr. Opin. Neurobiol. 14, 272–279 (2004).
Markus, A., Patel, T.D. & Snider, W.D. Neurotrophic factors and axonal growth. Curr. Opin. Neurobiol. 12, 523–531 (2002).
Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004).
Waites, C.L., Craig, A.M. & Garner, C.C. Mechanisms of vertebrate synaptogenesis. Annu. Rev. Neurosci. 28, 251–274 (2005).
Sanes, J.R. & Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
McMahan, U.J. et al. Agrin isoforms and their role in synaptogenesis. Curr. Opin. Cell Biol. 4, 869–874 (1992).
Gautam, M. et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 525–535 (1996).
Xiong, W.C. & Mei, L. An unconventional role of neurotransmission in synapse formation. Neuron 46, 521–523 (2005).
Brandon, E.P. et al. Aberrant patterning of neuromuscular synapses in choline acetyltransferase–deficient mice. J. Neurosci. 23, 539–549 (2003).
Misgeld, T. et al. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron 36, 635–648 (2002).
Schaeffer, L., de Kerchove d'Exaerde, A. & Changeux, J.P. Targeting transcription to the neuromuscular synapse. Neuron 31, 15–22 (2001).
Hamburger, V. Trophic interactions in neurogenesis: a personal historical account. Annu. Rev. Neurosci. 3, 269–278 (1980).
Ciani, L. & Salinas, P.C. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6, 351–362 (2005).
Hall, A.C., Lucas, F.R. & Salinas, P.C. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525–535 (2000).
Mathew, D. et al. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 310, 1344–1347 (2005).
Packard, M. et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 111, 319–330 (2002).
Luo, Z.G. et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 35, 489–505 (2002).
Wang, Z.Z. et al. Aberrant development of motor axons and neuromuscular synapses in MyoD-null mice. J. Neurosci. 23, 5161–5169 (2003).
Cadigan, K.M. & Nusse, R. Wnt signaling: a common theme in animal development. Genes Dev. 11, 3286–3305 (1997).
Haegel, H. et al. Lack of β-catenin affects mouse development at gastrulation. Development 121, 3529–3537 (1995).
Brault, V. et al. Inactivation of the β-catenin gene by Wnt1-Cre–mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001).
Miniou, P. et al. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 27, e27 (1999).
Schwander, M. et al. β1 integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell 4, 673–685 (2003).
Mao, X., Fujiwara, Y. & Orkin, S.H. Improved reporter strain for monitoring Cre recombinase–mediated DNA excisions in mice. Proc. Natl. Acad. Sci. USA 96, 5037–5042 (1999).
Gautam, M. et al. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 377, 232–236 (1995).
Zhang, B. et al. β-catenin regulates acetylcholine receptor clustering in muscle cells through interaction with rapsyn. J. Neurosci. 27, 3968–3973 (2007).
Lin, W. et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 1057–1064 (2001).
Yang, X. et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 399–410 (2001).
Fu, A.K. et al. Aberrant motor axon projection, acetylcholine receptor clustering and neurotransmission in cyclin-dependent kinase 5 null mice. Proc. Natl. Acad. Sci. USA 102, 15224–15229 (2005).
Drees, F., Pokutta, S., Yamada, S., Nelson, W.J. & Weis, W.I. α-catenin is a molecular switch that binds E-cadherin–β-catenin and regulates actin-filament assembly. Cell 123, 903–915 (2005).
Murase, S., Mosser, E. & Schuman, E.M. Depolarization drives β-catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35, 91–105 (2002).
Bamji, S.X. et al. Role of β-catenin in synaptic vesicle localization and presynaptic assembly. Neuron 40, 719–731 (2003).
Arber, S. et al. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23, 659–674 (1999).
Thaler, J. et al. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron 23, 675–687 (1999).
Huang, E.J. & Reichardt, L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Tao, H.W. & Poo, M. Retrograde signaling at central synapses. Proc. Natl. Acad. Sci. USA 98, 11009–11015 (2001).
Prakash, S., Caldwell, J.C., Eberl, D.F. & Clandinin, T.R. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat. Neurosci. 8, 443–450 (2005).
Jontes, J.D., Emond, M.R. & Smith, S.J. In vivo trafficking and targeting of N-cadherin to nascent presynaptic terminals. J. Neurosci. 24, 9027–9034 (2004).
Togashi, H. et al. Cadherin regulates dendritic spine morphogenesis. Neuron 35, 77–89 (2002).
Bozdagi, O., Valcin, M., Poskanzer, K., Tanaka, H. & Benson, D.L. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol. Cell. Neurosci. 27, 509–521 (2004).
Iwai, Y. et al. DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol. Cell. Neurosci. 19, 375–388 (2002).
Nuriya, M. & Huganir, R.L. Regulation of AMPA receptor trafficking by N-cadherin. J. Neurochem. 97, 652–661 (2006).
Lohof, A.M., Ip, N.Y. & Poo, M.M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363, 350–353 (1993).
Nguyen, Q.T., Parsadanian, A.S., Snider, W.D. & Lichtman, J.W. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science 279, 1725–1729 (1998).
Wang, T., Xie, K. & Lu, B. Neurotrophins promote maturation of developing neuromuscular synapses. J. Neurosci. 15, 4796–4805 (1995).
Bernstein, M. & Lichtman, J.W. Axonal atrophy: the retraction reaction. Curr. Opin. Neurobiol. 9, 364–370 (1999).
Koenen, M., Peter, C., Villarroel, A., Witzemann, V. & Sakmann, B. Acetylcholine receptor channel subtype directs the innervation pattern of skeletal muscle. EMBO Rep. 6, 570–576 (2005).
Lin, W. et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 46, 569–579 (2005).
Dong, X.P. et al. Shp2 is dispensable in the formation and maintenance of the neuromuscular junction. Neurosignals 15, 53–63 (2006).
McLachlan, E.M. & Martin, A.R. Non-linear summation of end-plate potentials in the frog and mouse. J. Physiol. (Lond.) 311, 307–324 (1981).
Acknowledgements
We are grateful to J. Melki and S. Arber for valuable mouse lines. This work was supported in part by grants from the US National Institutes of Health and the Muscular Dystrophy Association to L.M. and W.-C.X., National Natural Science Foundation of China (U0632007) and Program of Changjian Scholars and Innovative Research Team in University (IRT0731) to T.-M.G. T.-M.G. and L.M. are Chang Jiang Scholars.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Methods (PDF 682 kb)
Rights and permissions
About this article
Cite this article
Li, XM., Dong, XP., Luo, SW. et al. Retrograde regulation of motoneuron differentiation by muscle β-catenin. Nat Neurosci 11, 262–268 (2008). https://doi.org/10.1038/nn2053
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2053
This article is cited by
-
Synaptic retrograde regulation of the PKA-induced SNAP-25 and Synapsin-1 phosphorylation
Cellular & Molecular Biology Letters (2023)
-
Intrafusal-fiber LRP4 for muscle spindle formation and maintenance in adult and aged animals
Nature Communications (2023)
-
Synapse-specific Lrp4 mRNA enrichment requires Lrp4/MuSK signaling, muscle activity and Wnt non-canonical pathway
Cell & Bioscience (2021)
-
Elimination of glutamatergic transmission from Hb9 interneurons does not impact treadmill locomotion
Scientific Reports (2021)
-
Wnt antagonist FRZB is a muscle biomarker of denervation atrophy in amyotrophic lateral sclerosis
Scientific Reports (2020)