Abstract
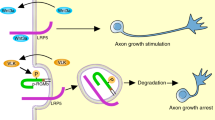
Eph receptors are activated by the autophosphorylation of tyrosine residues upon the binding of their ligands, the ephrins; however, the protein tyrosine phosphatases (PTPs) responsible for the negative regulation of Eph receptors have not been elucidated. Here, we identified protein tyrosine phosphatase receptor type O (Ptpro) as a specific PTP that efficiently dephosphorylates both EphA and EphB receptors as substrates. Biochemical analyses revealed that Ptpro dephosphorylates a phosphotyrosine residue conserved in the juxtamembrane region, which is required for the activation and signal transmission of Eph receptors. Ptpro thus seems to moderate the amount of maximal activation of Eph receptors. Using the chick retinotectal projection system, we show that Ptpro controls the sensitivity of retinal axons to ephrins and thereby has a crucial role in the establishment of topographic projections. Our findings explain the molecular mechanism that determines the threshold of the response of Eph receptors to ephrins in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 90, 403–404 (1997).
Poliakov, A., Cotrina, M. & Wilkinson, D.G. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell 7, 465–480 (2004).
Gale, N.W. et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 17, 9–19 (1996).
Himanen, J.P. et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat. Neurosci. 7, 501–509 (2004).
Zisch, A.H. et al. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses. Oncogene 19, 177–187 (2000).
Binns, K.L., Taylor, P.P., Sicheri, F., Pawson, T. & Holland, S.J. Phosphorylation of tyrosine residues in the kinase domain and juxtamembrane region regulates the biological and catalytic activities of Eph receptors. Mol. Cell. Biol. 20, 4791–4805 (2000).
Wybenga-Groot, L.E. et al. Structural basis for autoinhibition of the EphB2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell 106, 745–757 (2001).
Kullander, K. & Klein, R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 3, 475–486 (2002).
Knöll, B. & Drescher, U. Ephrin-As as receptors in topographic projections. Trends Neurosci. 25, 145–149 (2002).
Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 6, 462–475 (2005).
McLaughlin, T. & O'Leary, D.D. Molecular gradients and development of retinotopic maps. Annu. Rev. Neurosci. 28, 327–355 (2005).
Hansen, M.J., Dallal, G.E. & Flanagan, J.G. Retinal axon response to ephrin-As shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron 42, 717–730 (2004).
Hindges, R., McLaughlin, T., Genound, N., Henkemeyer, M. & O'Leary, D.D. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron 35, 475–487 (2002).
Shintani, T. et al. Large-scale identification and characterization of genes with asymmetric expression patterns in the developing chick retina. J. Neurobiol. 59, 34–47 (2004).
Andersen, J.N. et al. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 18, 8–30 (2004).
Ledig, M.M. et al. Expression of receptor tyrosine phosphatases during development of the retinotectal projection of the chick. J. Neurobiol. 39, 81–96 (1999).
Stepanek, L. et al. CRYP-2/cPTPRO is a neurite inhibitory repulsive guidance cue for retinal neurons in vitro. J. Cell Biol. 154, 867–878 (2001).
Ostman, A. & Bohmer, F.-D. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatases. Trends Cell Biol. 11, 258–266 (2001).
Flint, A.J., Tiganis, T., Barford, D. & Tonks, N.K. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94, 1680–1685 (1997).
Vielmetter, J., Stolze, B., Bonhoeffer, F. & Stuermer, C.A. In vitro assay to test differential substrate affinities of growing axons and migratory cells. Exp. Brain Res. 81, 283–287 (1990).
Hornberger, M.R. et al. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22, 731–742 (1999).
Cheng, H.J. & Flanagan, J.G. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell 79, 157–168 (1994).
Feldheim, D.A. et al. Topographic guidance labels in a sensory projection to the forebrain. Neuron 21, 1303–1313 (1998).
Chen, C.M. et al. Production and design of more effective avian replication-incompetent retroviral vectors. Dev. Biol. 214, 370–384 (1999).
Bell, E.J. & Brickell, P.M. Replication-competent retroviral vectors for expressing genes in avian cells in vitro and in vivo. Mol. Biotechnol. 7, 289–298 (1997).
Cheng, H.J., Nakamoto, M., Bergemann, A.D. & Flanagan, J.G. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell 82, 371–381 (1995).
Holash, J.A. et al. Reciprocal expression of the Eph receptor Cek5 and ligand(s) in the early retina. Dev. Biol. 182, 256–269 (1997).
Connor, R.J., Menzel, P. & Pasquale, E.B. Expression and tyrosine phosphorylation suggest multiple mechanisms in patterning of the visual system. Dev. Biol. 193, 21–35 (1998).
Johnson, K.G. & Van Vactor, D. Receptor protein tyrosine phosphatases in nervous system development. Physiol. Rev. 83, 1–24 (2003).
Hubbard, S. & Till, J.H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 69, 373–398 (2000).
Hubbard, S.R. Juxtamembrane autoinhibition in receptor tyrosine kinases. Nat. Rev. Mol. Cell Biol. 5, 464–470 (2004).
Eberhart, J. et al. Ephrin-A5 exerts positive or inhibitory effects on distinct subsets of EphA4-positive motor neurons. J. Neurosci. 24, 1070–1078 (2004).
Holmberg, J., Clarke, D.L. & Frisen, J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408, 203–206 (2000).
Maeda, N., Nishiwaki, T., Shintani, T., Hamanaka, H. & Noda, M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase ζ/RPTPβ, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM). J. Biol. Chem. 271, 21446–21452 (1996).
Meng, K. et al. Pleiotrophin signals increased tyrosine phosphorylation of β-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase β/ζ. Proc. Natl. Acad. Sci. USA 97, 2603–2608 (2000).
Kawachi, H., Fujikawa, A., Maeda, N. & Noda, M. Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase ζ/β by the yeast substrate-trapping system. Proc. Natl. Acad. Sci. USA 98, 6593–6598 (2001).
Maeda, N. et al. A receptor-like protein-tyrosine phosphatase PTPζ/RPTPβ binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPζ. J. Biol. Chem. 274, 12474–12479 (1999).
Hattori, M., Osterfield, M. & Flanagan, J.G. Regulated cleavage of a contact-mediated axon repellent. Science 289, 1360–1365 (2000).
Janes, P.W. et al. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123, 291–304 (2005).
Marston, D.J., Dickinson, S. & Nobes, C.D. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat. Cell Biol. 5, 879–888 (2003).
Zimmer, M., Palmer, A., Kohler, J. & Klein, R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 5, 869–878 (2003).
Rosentreter, S.M. et al. Response of retinal ganglion cell axons to striped linear gradients of repellent guidance molecules. J. Neurobiol. 37, 541–562 (1998).
Walter, J., Müller, B. & Bonhoeffer, F. Axonal guidance by an avoidance mechanism. J. Physiol. (Paris) 84, 104–110 (1990).
Wang, H.U. & Anderson, D.J. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 18, 383–396 (1997).
Yuasa-Kawada, J. et al. Axonal morphogenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. Eur. J. Neurosci. 17, 2329–2343 (2003).
Yuasa, J., Hirano, S., Yamagata, M. & Noda, M. Visual projection map specified by topographic expression of transcription factors in the retina. Nature 382, 632–635 (1996).
Sakuta, H. et al. Ventroptin: A BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science 293, 111–115 (2001).
Takahashi, H., Shintani, T., Sakuta, H. & Noda, M. CBF1 controls the retinotectal topographical map along the anteroposterior axis through multiple mechanisms. Development 130, 5203–5215 (2003).
Acknowledgements
We thank C.L. Cepko (Harvard Medical School, Boston) for providing the RIAS vector and F. Bonhoeffer (Max-Planck-Institute for Developmental Biology, Tübingen) for the silicon matrix used to produce the ephrin-A2-Fc stripe. We also thank Y. Ayabe and M. Gotoh for technical assistance and A. Kodama for secretarial assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Core Research for Evolutional Science and Technology (CREST) program of the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Expression analysis of Ptpro in the developing chick retina. (PDF 58 kb)
Supplementary Fig. 2
Specific dephosphorylation of Eph receptors by Ptpro. (PDF 33 kb)
Supplementary Fig. 3
Inhibition of ephrin-induced tyrosine phosphorylation of Eph receptors by Ptpro in PC-3 prostrate epithelial cells expressing EphA2 endogenously. (PDF 28 kb)
Supplementary Fig. 4
Ptprz exerts no effect on the sensitivity of chick retinal axons to ephrin-A2-Fc. (PDF 32 kb)
Supplementary Fig. 5
Improvement of transfection and expression of the transgene of the RIAS vector on simultaneous electroporation of the RCAS vector. (PDF 28 kb)
Supplementary Fig. 6
Retinotectal projection at E19 in embryos electroporated with RCAS/PtproδN(DA). (PDF 57 kb)
Rights and permissions
About this article
Cite this article
Shintani, T., Ihara, M., Sakuta, H. et al. Eph receptors are negatively controlled by protein tyrosine phosphatase receptor type O. Nat Neurosci 9, 761–769 (2006). https://doi.org/10.1038/nn1697
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1697
This article is cited by
-
A slit-diaphragm-associated protein network for dynamic control of renal filtration
Nature Communications (2022)
-
The endosomal sorting adaptor HD-PTP is required for ephrin-B:EphB signalling in cellular collapse and spinal motor axon guidance
Scientific Reports (2019)
-
Eph receptor signalling: from catalytic to non-catalytic functions
Oncogene (2019)
-
PTPRJ Inhibits Leptin Signaling, and Induction of PTPRJ in the Hypothalamus Is a Cause of the Development of Leptin Resistance
Scientific Reports (2017)
-
PTPROt-mediated regulation of p53/Foxm1 suppresses leukemic phenotype in a CLL mouse model
Leukemia (2015)