Abstract
The axon guidance cue netrin is importantly involved in neuronal development. DCC (deleted in colorectal cancer) is a functional receptor for netrin and mediates axon outgrowth and the steering response. Here we show that different regions of the intracellular domain of DCC directly interacted with the tyrosine kinases Src and focal adhesion kinase (FAK). Netrin activated both FAK and Src and stimulated tyrosine phosphorylation of DCC. Inhibition of Src family kinases reduced DCC tyrosine phosphorylation and blocked both axon attraction and outgrowth of neurons in response to netrin. Mutation of the tyrosine phosphorylation residue in DCC abolished its function of mediating netrin-induced axon attraction. On the basis of our observations, we suggest a model in which DCC functions as a kinase-coupled receptor, and FAK and Src act immediately downstream of DCC in netrin signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Serafini, T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994).
Kennedy, T.E., Serafini, T., de la Torre, J.R. & Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435 (1994).
Tessier-Lavigne, M. & Goodman, C.S. The molecular biology of axon guidance. Science 274, 1123–1133 (1996).
Culotti, J.G. & Merz, D.C. DCC and netrins. Curr. Opin. Cell Biol. 10, 609–613 (1998).
Chan, S.S. et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87, 187–195 (1996).
Keino-Masu, K. et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 (1996).
Kolodziej, P.A. et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87, 197–204 (1996).
Fearon, E.R. et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 247, 49–56 (1990).
Fazeli, A. et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386, 796–804 (1997).
Leung-Hagesteijn, C. et al. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell 71, 289–299 (1992).
Hamelin, M., Zhou, Y., Su, M.W., Scott, I.M. & Culotti, J.G. Expression of the UNC-5 guidance receptor in the touch neurons of C. elegans steers their axons dorsally. Nature 364, 327–330 (1993).
Hong, K. et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927–941 (1999).
Leonardo, E.D. et al. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386, 833–838 (1997).
Tong, J. et al. Netrin stimulates tyrosine phosphorylation of the UNC-5 family of netrin receptors and induces Shp2 binding to the RCM cytodomain. J. Biol. Chem. 276, 40917–40925 (2001).
Parsons, J.T., Martin, K.H., Slack, J.K., Taylor, J.M. & Weed, S.A. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19, 5606–5613 (2000).
Schaller, M.D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta 1540, 1–21 (2001).
Schlaepfer, D.D., Hauck, C.R. & Sieg, D.J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71, 435–478 (1999).
Lowell, C.A. & Soriano, P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 10, 1845–1857 (1996).
Thomas, S.M. & Brugge, J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13, 513–609 (1997).
Schaller, M.D., Borgman, C.A. & Parsons, J.T. Autonomous expression of a noncatalytic domain of the focal adhesion–associated protein tyrosine kinase pp125FAK. Mol. Cell. Biol. 13, 785–791 (1993).
Hanke, J.H. et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 (1996).
Calalb, M.B., Polte, T.R. & Hanks, S.K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 15, 954–963 (1995).
Sperber, B.R. et al. A unique role for Fyn in CNS myelination. J. Neurosci. 21, 2039–2047 (2001).
Bockholt, S.M. & Burridge, K. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes- mice. Cell Adhes. Commun. 3, 91–100 (1995).
Cary, L.A. & Guan, J.L. Focal adhesion kinase in integrin-mediated signaling. Front. Biosci. 4, D102–D113 (1999).
Richardson, A., Malik, R.K., Hildebrand, J.D. & Parsons, J.T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol. Cell. Biol. 17, 6906–6914 (1997).
Tessier-Lavigne, M., Placzek, M., Lumsden, A.G., Dodd, J. & Jessell, T.M. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature 336, 775–778 (1988).
Ming, G.L. et al. cAMP-dependent growth cone guidance by netrin-1. Neuron 19, 1225–1235 (1997).
Liu, G.B.H. et al. Netrin requires the focal adhesion kinase and the Src family kinases to induce axon outgrowth and to attract axons. Nat. Neurosci. 7, 1222–1232 (2004).
Ren, X.G.M. et al. Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 7, 1204–1212 (2004).
Chang, C., Yu, T.W., Bargmann, C.I. & Tessier-Lavigne, M. Inhibition of netrin-mediated axon attraction by a receptor protein tyrosine phosphatase. Science 305, 103–106 (2004).
Stein, E. & Tessier-Lavigne, M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291, 1928–1938 (2001).
Ming, G. et al. Phospholipase C-γ and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron 23, 139–148 (1999).
Li, X. et al. The adaptor protein Nck-1 couples the netrin-1 receptor DCC (Deleted in Colorectal Cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J. Biol. Chem. 277, 37788–37797 (2002).
Li, X., Saint-Cyr-Proulx, E., Aktories, K. & Lamarche-Vane, N. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E–115 neuroblastoma cells. J. Biol. Chem. 277, 15207–15214 (2002).
Shekarabi, M. & Kennedy, T.E. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell. Neurosci. 19, 1–17 (2002).
Forcet, C. et al. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature 417, 443–447 (2002).
Ming, G.L. et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature 417, 411–418 (2002).
Graef, I.A. et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell 113, 657–670 (2003).
Cooper, L.A., Shen, T.L. & Guan, J.L. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol. Cell. Biol. 23, 8030–8041 (2003).
Tabti, N. & Poo, M.-M. Culturing spinal cord neurons and muscle cells from Xenopus embryos. in Culturing Nerve Cells (eds. Banker, G. & Goslin, K.) 137–154 (MIT Press, Cambridge, Massachusetts, USA, 1991).
Schaller, M.D. et al. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14, 1680–1688 (1994).
Acknowledgements
We thank B. Kruger for critical reading of the manuscript, T. Zhu for technical assistance, and W. Xiong for communicating unpublished information. This work is supported by grants from the National Institutes of Health (K.-L.G.), a Rackham Graduate Fellowship (H.V.) and the Pharmocological Sciences Training Program (GMO7767 to J.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
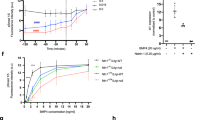
DCC tyrosine phosphorylation and interaction with FAK and Src. (a) Netrin induces time- and dose-dependent tyrosine phosphorylation of DCC. HEK 293 cells were transfected with the indicated plasmids, stimulated with netrin and tyrosine phosphorylation of immunoprecipitated DCC was observed as a function of time (0-30 min) and concentration (0-2 μg/ml). (b) In vitro pulldown of FAK by GST-DCC. HEK293 cells were transfected with HA-FAK and lysates were incubated with GST and GST-DCC intracellular domain produced from HEK293 cells (lanes 1-2) or E.coli (lanes 3-4). The presence of FAK was determined by α-HA. Shown also is a Coomassie blue stained membrane of the GST-DCC protein. (c) Dose dependent inhibition of DCC tyrosine phosphorylation by PP2. PP2 was used at various concentrations for 60 min and the phosphorylation state of immunoprecipitated DCC was determined by phosphotyrosine IB. (d) Netrin-DCC induction of FAK site specific phosphorylation and inhibition by PP2. FAK was isolated by IP and probed with the indicated FAK phospho-specific antibodies. The presence of PP2 was indicated. (e) In vitro pulldown of Src and FAK by GST-DCC. Src and HA-FAK proteins immunoprecipitated from transfected 293 cell under high stringency conditions were incubated with GST-DCC purified from E.coli. The complex was precipitated with anti-Src or anti-HA (for HA-FAK) and the presence of DCC was detected by IB. (f) Netrin does not affect the interaction of DCC with FAK. The experiment was performed as described in Fig. 2f. (g) Fyn interacts with DCC through the same mechanism as Src. HEK 293 cells were transfected with indicated plasmids. Src or Fyn was immunoprecipitated and the presence of DCC in IP was determined by IB. (h) PP2 has no effects on the interaction of DCC and FAK. HEK 293 cells were transfected with the indicated plasmids and where treated with PP2. HA-FAK was immunoprecipitated and the presence of DCC in the IP was determined by IB. (JPG 92 kb)
Supplementary Fig. 2
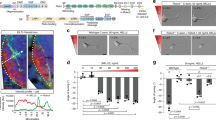
A model for FAK and Src in netrin signaling. FAK interacts via its CTD with the P3 domain of DCC. Src interacts in part via its SH3 domain to the P1400XXP motif in DCC and in part via its SH2 domain to phosphorylated Y397 on FAK. Src phosphorylates Y1420 in DCC. Note that molecules except the intracellular domain of DCC are not drawn to scale. CTD, C-terminal domain; NTD, N-terminal domain of FAK. (GIF 40 kb)
Rights and permissions
About this article
Cite this article
Li, W., Lee, J., Vikis, H. et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci 7, 1213–1221 (2004). https://doi.org/10.1038/nn1329
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1329
This article is cited by
-
Asymmetric activity of NetrinB controls laterality of the Drosophila brain
Nature Communications (2023)
-
Netrin-1 induces the anti-apoptotic and pro-survival effects of B-ALL cells through the Unc5b-MAPK axis
Cell Communication and Signaling (2022)
-
Investigating regions of shared genetic variation in attention deficit/hyperactivity disorder and major depressive disorder: a GWAS meta-analysis
Scientific Reports (2021)
-
Ephrin-A5 potentiates netrin-1 axon guidance by enhancing Neogenin availability
Scientific Reports (2019)
-
The binding of DCC-P3 motif and FAK-FAT domain mediates the initial step of netrin-1/DCC signaling for axon attraction
Cell Discovery (2018)