Abstract
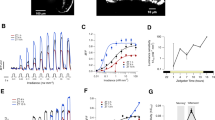
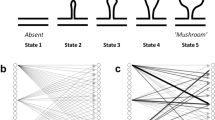
Naturally occurring rearrangements of synaptic terminals are common in the nervous systems of young mammals, but little is known about their incidence in adults. Using transgenic mice that express yellow fluorescent protein (YFP) in axons, we repeatedly imaged nerve terminals in the parasympathetic submandibular ganglion. We found that the pattern of synaptic branches underwent significant rearrangements over several weeks in young adult mice. In older mice, rearrangements were less common, and synaptic patterns on individual neurons were recognizable for many months to years. Axonal branches frequently retracted or extended on a time scale of minutes in young adult mice, but seldom in mature animals. These results provide direct evidence for a decrease in plasticity of interneuronal connections as animals make the transition from young adulthood to middle age. The long-term stability of synaptic patterns could provide a structural basis for the persistence of memory in the adult nervous system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hubel, D.H., Wiesel, T.N. & LeVay, S. Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 278, 377–409 (1977).
Rakic, P., Bourgeois, J.P., Eckenhoff, M.F., Zecevic, N. & Goldman-Rakic, P.S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 11, 232–235 (1986).
Katz, L.C. & Shatz, C.J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996)
Lichtman, J.W. & Colman, H. Synapse elimination and indelible memory. Neuron 25, 269–278 (2000).
Balice-Gordon, R.J. & Lichtman, J.W. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature 372, 519–524 (1994).
Darian-Smith, C. & Gilbert, C.D. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 368, 737–740 (1994).
Jones, T.A., Klintsova, A.Y., Kilman, V.L., Sirevaag, A.M. & Greenough, W.T. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol. Learn. Mem. 68, 13–20 (1997).
Kilgard, M.P. & Merzenich, M.M. Plasticity of temporal information processing in the primary auditory cortex. Nat. Neurosci. 1, 727–731 (1998).
Florence, S.L., Taub, H.B. & Kaas, J.H. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282, 1117–1121 (1998).
Jones, E.G. & Pons, T.P. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science 282, 1121–1125 (1998).
Bao, S., Chan, V.T. & Merzenich, M.M. Cortical remodeling induced by activity of ventral tegmental dopamine neurons. Nature 412, 79–83 (2001).
Knott, G.W., Quairiaux, C., Genoud, C. & Welker, E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, 265–273 (2002).
Bertoni-Freddari, C. et al. Synaptic structural dynamics and aging. Gerontology 42, 170–180 (1996).
Desai, N.S., Cudmore, R.H., Nelson, S.B. & Turrigiano, G.G. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat. Neurosci. 5, 783–789 (2002).
Sawaki, L., Yaseen, Z., Kopylev, L. & Cohen, L.G. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann. Neurol. 53, 521–524 (2003).
Grutzendler, J., Kasthuri, N. & Gan, W.B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Daw, N.W. Critical periods and amblyopia. Arch. Ophthalmol. 116, 502–505 (1998).
Lichtman, J.W., Magrassi, L. & Purves, D. Visualization of neuromuscular junctions over periods of several months in living mice. J. Neurosci. 7, 1215–1222 (1987).
Balice-Gordon, R.J. & Lichtman, J.W. In vivo visualization of the growth of pre- and postsynaptic elements of neuromuscular junctions in the mouse. J. Neurosci. 10, 894–908 (1990).
Walsh, M.K. & Lichtman, J.W. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron 37, 67–73 (2003).
Purves, D, & Hadley, R.D. Changes in the dendritic branching of adult mammalian neurones revealed by repeated imaging in situ. Nature 315, 404–406 (1985).
Purves, D., Hadley, R.D. & Voyvodic, J.T. Dynamic changes in the dendritic geometry of individual neurons visualized over periods of up to three months in the superior cervical ganglion of living mice. J. Neurosci. 6, 1051–1060 (1986).
Purves, D., Voyvodic, J.T., Magrassi, L. & Yawo, H. Nerve terminal remodeling visualized in living mice by repeated examination of the same neuron. Science 238, 1122–1126 (1987).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Trachtenberg, J.T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Purves, D. & Lichtman, J.W. Synaptic sites on reinnervated nerve cells visualized at two different times in living mice. J. Neurosci. 7, 1492–1497 (1987).
Lichtman, J.W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J. Physiol. 273, 155–177 (1977).
Snider, W.D. The dendritic complexity and innervation of submandibular neurons in five species of mammals. J. Neurosci. 7, 1760–1768 (1987).
Snider, W.D. & Lichtman, J.W. Are neurotrophins synaptotrophins? Mol. Cell. Neurosci. 7, 433–442 (1996).
McEwen, B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122 (1999).
Zhang, W. & Benson D.L. Stages of synapse development defined by dependence on F-actin. J. Neurosci. 21, 5169–5181 (2001).
Eaton, B.A., Fetter, R.D. & Davis, G.W. Dynactin is necessary for synapse stabilization. Neuron 34, 729–741 (2002).
Bruses, J.L. Cadherin-mediated adhesion at the interneuronal synapse. Curr. Opin. Cell Biol. 12, 593–597 (2000).
Sanes, J.R. & Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Acknowledgements
We thank H. Suk-Woo for help with data analysis. This work was supported by grants from the US National Institutes of Health (NIH) to J.W.L. and J.R.S. W-B.G. and E.K. were supported by grants to W-B.G. from the NIH (R01 NS41846-01) and the Ellison Foundation. Support from the Bakewell NeuroImaging Fund is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gan, WB., Kwon, E., Feng, G. et al. Synaptic dynamism measured over minutes to months: age-dependent decline in an autonomic ganglion. Nat Neurosci 6, 956–960 (2003). https://doi.org/10.1038/nn1115
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1115
This article is cited by
-
Multiple Phases of Climbing Fiber Synapse Elimination in the Developing Cerebellum
The Cerebellum (2018)
-
Synapse microarray identification of small molecules that enhance synaptogenesis
Nature Communications (2011)
-
Experience-dependent structural synaptic plasticity in the mammalian brain
Nature Reviews Neuroscience (2009)
-
Rapid and modifiable neurotransmitter receptor dynamics at a neuronal synapse in vivo
Nature Neuroscience (2008)
-
A technicolour approach to the connectome
Nature Reviews Neuroscience (2008)