Abstract
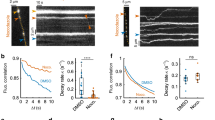
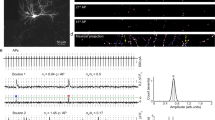
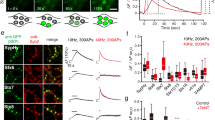
Sustained release of neurotransmitter depends upon the recycling of synaptic vesicles. Until now, it has been assumed that vesicle recycling is regulated by signals from the presynaptic bouton alone, but results from rat hippocampal neurons reported here indicate that this need not be the case. Fluorescence imaging and pharmacological analysis show that a nitric oxide (NO) signal generated postsynaptically can regulate endocytosis and at least one later step in synaptic vesicle recycling. The proposed retrograde pathway involves an NMDA receptor (NMDAR)-dependent postsynaptic production of NO, diffusion of NO to a presynaptic site, and a cGMP-dependent increase in presynaptic phosphatidylinositol 4,5-biphosphate (PIP2). These results indicate that the regulation of synaptic vesicle recycling may integrate a much broader range of neural activity signals than previously recognized, including postsynaptic depolarization and the activation of NMDARs at both immediate and nearby postsynaptic active zones.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tao, H.W. & Poo, M. Retrograde signaling at central synapses. Proc. Natl. Acad. Sci. USA 98, 11009–11015 (2001).
Wilson, R.I. & Nicoll, R.A. Endocannabinoid signaling in the brain. Science 296, 678–682 (2002).
Prast, H. & Philippu, A. Nitric oxide as modulator of neuronal function. Prog. Neurobiol. 64, 51–68 (2001).
Arancio, O., Kandel, E.R. & Hawkins, R.D. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cGMP in cultured hippocampal neurons. Nature 376, 74–80 (1995).
Meffert, M.K., Calakos, N.C., Scheller, R.H. & Schulman, H. Nitric oxide modulates synaptic vesicle docking/fusion reactions. Neuron 16, 1229–1236 (1996).
Sporns, O. & Jenkinson, S. Potassium ion- and nitric oxide-induced exocytosis from populations of hippocampal synapses during synaptic maturation in vitro. Neuroscience 80, 1057–1073 (1997).
Klyachko, V.A., Ahern, G.P. & Jackson, M.B. cGMP-mediated facilitation in nerve terminals by enhancement of the spike after hyperpolarization. Neuron 31, 1015–1025 (2001).
Micheva, K.D., Holz, R.W. & Smith, S.J. Regulation of presynaptic phosphatidy-linositol 4,5-biphosphate by neuronal activity. J. Cell Biol. 154, 355–368 (2001).
Cremona, O. & De Camilli, P. Phosphoinositides in membrane traffic at the synapse. J. Cell Sci. 114, 1041–1052 (2001).
Martin, T.F.J. PI(4,5)P(2) regulation of surface membrane traffic. Curr. Opin. Cell Biol. 13, 493–499 (2001).
Osborne, S.L., Meunier, F.A. & Schiavo, G. Phosphoinositides as key regulators of synaptic function. Neuron 32, 9–12 (2001).
Rao, A., Kim, E., Sheng, M. & Craig, A.-M. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J. Neurosci. 18, 1217–1229 (1998).
Wendland, B. et al. Existence of nitric oxide synthase in rat hippocampal pyramidal cells. Proc. Natl. Acad. Sci. USA 91, 2151–2155 (1994).
Burette, A., Zabel, U., Weinberg, R.J., Schmidt, H.H.H.W. & Valtschanoff, J.G. Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J. Neurosci. 22, 8961–8970 (2002).
Son, H. et al. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell 87, 1015–1023 (1996).
De Camilli, P., Slepnev, V.I., Shupliakov, O. & Brodin, L. Synaptic vesicle endocytosis. in Synapses (eds. Cowan, M.W., Sudhof, T.C. & Stevens, C.F.) 217–274 (The Johns Hopkins Univ. Press, Baltimore, MD, 2001).
Li, Z. & Murthy, V.N. Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses. Neuron 31, 593–605 (2001).
Betz, W.J., Mao, F. & Bewick, G.S. Activity-dependent fluorescent staining and destaining of living motor nerve terminals. J. Neurosci. 12, 363–375 (1992).
Ryan, T.A. Presynaptic imaging techniques. Curr. Opin. Neurobiol. 11, 544–549 (2001).
Khvotchev, M. & Südhof, T. Newly synthesized phosphatidylinositol phosphates are required for synaptic norepinephrine but not glutamate or γ-aminobutyric acid (GABA) release. J. Biol. Chem. 273, 21451–21454 (1998).
Varnai, P. & Balla, T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143, 501–510 (1998).
Paterson, H.F. et al. Phospholipase C delta 1 requires a pleckstrin homology domain for interaction with the plasma membrane. Biochem. J. 312, 661–666 (1995).
Ryan, T.A. & Smith, S.J. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron 14, 983–989 (1995).
Hokin, L. & Hokin, M.R. Biochim. Biophys. Acta 84, 563–575 (1964).
Eichberg, J. & Dawson, R.M. Polyphosphoinositides in myelin. Biochem. J. 96, 644–650 (1965).
Klingauf, J., Kavalali, E.T. & Tsien, R.W. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature 394, 581–585 (1998).
Sankaranarayanan, S. & Ryan, T.A. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat. Neurosci. 4, 129–136 (2001).
Berridge, M.J., Lipp, P. & Bootman, M.D. The versatility and universatility of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000).
Wu, L., Bauer, C.S., Zhen, X., Xie, C. & Yang, J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature 419, 947–952 (2002).
Gaidarov, I. & Keen, J.H. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J. Cell Biol. 146, 755–764 (1999).
Ford, M.G.J. et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291, 1051–1055 (2001).
Itoh, T. et al. Role of the ENTH domain in phosphatidylinositol-4,5,-biphosphate binding and endocytosis. Science 291, 1047–1051 (2001).
Lin, H.C. & Gilman, A.G. Regulation of dynamin I GTPase activity by G protein beta gamma subunits and phosphatidylinositol 4,5-biphosphate. J. Biol. Chem. 271, 27979–27982 (1996).
Zheng, J. et al. Identification of the binding site for acidic phospholipids on the PH domain of dynamin: Implications for stimulation of GTPase activity. J. Mol. Biol. 255, 14–21 (1996).
Cremona, O. et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179–188 (1999).
Rozelle, A.L. et al. Phosphatidylinositol 4,5-biphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 10, 311–320 (2000).
Wenk, M.R. et al. PIP kinase Iγ is the major PI(4,5)P2 synthesizing enzyme at the synapse. Neuron 32, 79–88 (2001).
Guo, J. et al. Phosphatidylinositol 4-kinase type IIα is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc. Natl. Acad. Sci. USA 100, 3995–4000 (2003).
McPherson, P.S. et al. A presynaptic inositol-5-phosphatase. Nature 379, 353–357 (1996).
Clementi, E et al. Nitric oxide action on growth factor-elicited signals. Phosphoinositide hydrolysis and [Ca2+]i responses are negatively modulated via a cGMP-dependent protein kinase I pathway. J. Biol. Chem. 270, 22277–22282 (1995).
Goslin, K., Asmussen, H. & Banker, G. Rat hippocampal neurons in low-density culture. in Culturing Nerve Cells (eds. Banker, G. & Goslin, K.) 339–370 (MIT Press, Cambridge, Massachusetts, 1998).
Xia, Z., Dudek, H., Miranti, C.K. & Greenberg, M.E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996).
Acknowledgements
We thank R.W. Aldrich and the members of the Smith lab for critical reading of the manuscript and Y. Gedde for technical assistance. This work was supported by grants from the US National Institutes of Health and a gift from the Vincent Coates Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Micheva, K., Buchanan, J., Holz, R. et al. Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat Neurosci 6, 925–932 (2003). https://doi.org/10.1038/nn1114
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1114
This article is cited by
-
Molecular and neural mechanisms regulating sexual motivation of virgin female Drosophila
Cellular and Molecular Life Sciences (2021)
-
Putting a brake on synaptic vesicle endocytosis
Cellular and Molecular Life Sciences (2017)
-
TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P2
Nature Communications (2014)
-
pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity
Nature Neuroscience (2012)
-
A mathematical model for astrocytes mediated LTP at single hippocampal synapses
Journal of Computational Neuroscience (2012)