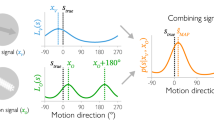
Abstract
Direction-selective neurons in the middle temporal visual area (MT) are crucially involved in motion perception, although it is not known exactly how the activity of these neurons is interpreted by the rest of the brain. Here we report that in a two-alternative task, the activity of MT neurons is interpreted as evidence for one direction and against the other. We measured the speed and accuracy of decisions as rhesus monkeys performed a direction-discrimination task. On half of the trials, we stimulated direction-selective neurons in area MT, thereby causing the monkeys to choose the neurons' preferred direction more often. Microstimulation quickened decisions in favor of the preferred direction and slowed decisions in favor of the opposite direction. Even on trials in which microstimulation did not induce a preferred direction choice, it still affected response times. Our findings suggest that during the formation of a decision, sensory evidence for competing propositions is compared and accumulates to a decision-making threshold.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vickers, D. Evidence for an accumulator model of psychophysical discrimination. Ergonomics 13, 37–58 (1970).
Luce, R.D. Response Times (Oxford Univ. Press, New York, 1986).
Link, S.W. The Wave Theory of Difference and Similarity (Erlbaum, Hillsdale, 1992).
Ratcliff, R. & Rouder, J.N. Modeling response times for two-choice decisions. Psychol. Sci. 9, 347–356 (1998).
Reddi, B.A. & Carpenter, R.H. The influence of urgency on decision time. Nat. Neurosci. 3, 827–830 (2000).
Usher, M. & McClelland, J.L. The time course of perceptual choice: the leaky, competing accumulator model. Psychol. Rev. 108, 550–592 (2001).
Aminoff, M.J. & Goodin, D.S. The decision to make a movement: neurophysiological insights. Can. J. Neurol. Sci. 24, 181–190 (1997).
Schall, J.D. & Bichot, N.P. Neural correlates of visual and motor decision processes. Curr. Opin. Neurobiol. 8, 211–217 (1998).
Glimcher, P.W. Making choices: the neurophysiology of visual-saccadic decision making. Trends Neurosci. 24, 654–659 (2001).
Romo, R. & Salinas, E. Touch and go: decision-making mechanisms in somatosensation. Annu. Rev. Neurosci. 24, 107–137 (2001).
Platt, M.L. Neural correlates of decisions. Curr. Opin. Neurobiol. 12, 141–148 (2002).
Romo, R. & Salinas, E. Flutter discrimination: neural codes, perception, memory and decision making. Nat. Rev. Neurosci. 4, 203–218 (2003).
Newsome, W.T., Britten, K.H. & Movshon, J.A. Neural correlates of a perceptual decision. Nature 341, 52–54 (1989).
Britten, K.H., Newsome, W.T., Shadlen, M.N., Celebrini, S. & Movshon, J.A. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis. Neurosci. 13, 87–100 (1996).
Shadlen, M.N. & Newsome, W.T. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 86, 1916–1936 (2001).
Gold, J.I. & Shadlen, M.N. Representation of a perceptual decision in developing oculomotor commands. Nature 404, 390–394 (2000).
Gold, J.I. & Shadlen, M.N. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J. Neurosci. 23, 632–651 (2003).
Roitman, J.D. & Shadlen, M.N. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J. Neurosci. 22, 9475–9489 (2002).
Salzman, C.D., Murasugi, C.M., Britten, K.H. & Newsome, W.T. Microstimulation in visual area MT: effects on direction discrimination performance. J. Neurosci. 12, 2331–2355 (1992).
Newsome, W.T. & Paré, E.B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J. Neurosci. 8, 2201–2211 (1988).
Rudolph, K. & Pasternak, T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cereb. Cortex 9, 90–100 (1999).
Albright, T.D. Cortical processing of visual motion. Rev. Oculomot. Res. 5, 177–201 (1993).
Cook, E.P. & Maunsell, J.H. Dynamics of neuronal responses in macaque MT and VIP during motion detection. Nat. Neurosci. 5, 985–994 (2002).
Celebrini, S. & Newsome, W.T. Microstimulation of extrastriate area MST influences performance on a direction discrimination task. J. Neurophysiol. 73, 437–448 (1995).
Laming, D.R.J. Subjective probability in choice-reaction experiments. J. Math. Psych. 6, 81–120 (1969).
Carpenter, R.H. & Williams, M.L. Neural computation of log likelihood in control of saccadic eye movements. Nature 377, 59–62 (1995).
Hanes, D.P. & Schall, J.D. Neural control of voluntary movement initiation. Science 274, 427–430 (1996).
Heeger, D.J., Boynton, G.M., Demb, J.B., Seidemann, S. & Newsome, W.T. Motion opponency in visual cortex. J. Neurosci. 19, 7162–7174 (1999).
Qian, N., Andersen, R.A. & Adelson, E.H. Transparent motion perception as detection of unbalanced motion signals. I. Psychophysics. J. Neurosci. 14, 7357–7366 (1994).
Qian, N. & Andersen, R.A. Transparent motion perception as detection of unbalanced motion signals. II. Physiology. J. Neurosci. 14, 7367–7380 (1994).
Britten, K.H., Shadlen, M.N., Newsome, W.T. & Movshon, J.A. Responses of neurons in macaque MT to stochastic motion signals. Vis. Neurosci. 10, 1157–1169 (1993).
Pantle, A. Motion aftereffect magnitude as a measure of the spatiotemporal response properties of direction-sensitive analyzers. Vision Res. 14, 1229–1236 (1974).
Levinson, E. & Sekuler, R. The independence of channels in human vision selective for direction of movement. J. Physiol. 250, 347–366 (1975).
Adelson, E.H. & Bergen, J.R. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Am. A2, 284–299 (1985).
Snowden, R.J., Treue, S., Erickson, R.G. & Andersen, R.A. The response of area MT and V1 neurons to transparent motion. J. Neurosci. 11, 2768–2785 (1991).
Van Wezel, R.J.A. & Britten, K.H. Motion adaptation in area MT. J. Neurophysiol. 88, 3469–3476 (2002).
Churchland, M.M. & Lisberger, S.G. Shifts in the population response in the middle temporal visual area parallel perceptual and motor illusions produced by apparent motion. J. Neurosci. 21, 9387–9402 (2001).
Horwitz, G.D. & Newsome, W.T. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J. Neurophysiol. 86, 2543–2558 (2001).
Kim, J.N. & Shadlen, M.N. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat. Neurosci. 2, 176–185 (1999).
Good, I.J. Studies in the history of probability and statistics: A.M. Turing's statistical work in World War II. Biometrika 66, 393–396 (1979).
Gold, J.I. & Shadlen, M.N. Neural computations that underlie decisions about sensory stimuli. Trends Cogn. Sci. 5, 10–16 (2001).
Wald, A. Sequential Analysis (Wiley, New York, 1947).
Van Essen, D.C. et al. An integrated software suite for surface-based analyses of cerebral cortex. J. Am. Med. Inform. Assoc. 8, 510–511 (2001).
Van Essen, D.C. Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr. Opin. Neurobiol. 12, 574–579 (2002).
Brainard, D.H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Pelli, D.G. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442 (1997).
Meeker, W.Q. & Escobar, L.A. Statistical Methods for Reliability Data (John Wiley & Sons, New York, 1998).
Press, W.H., Teukolsky, S.A., Vetterling, W.T., Flannery, B.P. Numerical Recipes in C 666–670 (Cambridge University Press, Cambridge, 1992).
Green, D.M. & Swets, J.A. Signal Detection Theory and Psychophysics (John Wiley & Sons, New York, 1966).
Acknowledgements
This study was supported by the ARCS Foundation, the Deutsche Forschungsgemeinschaft (DI 819/1-1), the Howard Hughes Medical Institute, the National Center for Research Resources (RR00166), the National Eye Institute (EY11378) and Poncin. We thank L. Jasinski and M. Mihali for technical assistance and J. Gold, A. Huk and J. Palmer for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ditterich, J., Mazurek, M. & Shadlen, M. Microstimulation of visual cortex affects the speed of perceptual decisions. Nat Neurosci 6, 891–898 (2003). https://doi.org/10.1038/nn1094
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1094
This article is cited by
-
Accounting for endogenous effects in decision-making with a non-linear diffusion decision model
Scientific Reports (2023)
-
Neural Control of Action Selection Among Innate Behaviors
Neuroscience Bulletin (2022)
-
Causal role for the primate superior colliculus in the computation of evidence for perceptual decisions
Nature Neuroscience (2021)
-
Breaking Deadlocks: Reward Probability and Spontaneous Preference Shape Voluntary Decisions and Electrophysiological Signals in Humans
Computational Brain & Behavior (2021)
-
Evidence accumulation during perceptual decisions in humans varies as a function of dorsal frontoparietal organization
Nature Human Behaviour (2020)