Abstract
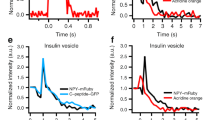
Ca2+ microdomains that form during the opening of voltage-gated Ca2+ channels have been implicated in regulating the kinetics of hormone and transmitter release. Direct assessment of the interaction between a single Ca2+ microdomain and a single secretory vesicle has been impossible because of technical limitations. Using evanescent field imaging of near-membrane micromolar Ca2+ concentration ([Ca2+]) and fluorescently labeled vesicles, we have observed exocytosis of individual chromaffin dense-core vesicles that was triggered by Ca2+ microdomains. Ca2+ microdomains selectively triggered the release of vesicles that were docked within 300 nm. Not all vesicles exposed to a Ca2+ microdomain were released, indicating that some vesicles are docked but are not ready for release. In addition to its established role as a trigger for release, elevated near-membrane [Ca2+] reduced the distance between docked vesicles and Ca2+ entry sites. Our results suggest a new mechanism for stimulation-dependent facilitation of exocytosis, whereby vesicles are moved closer to Ca2+ entry sites, thereby increasing a Ca2+ microdomain's efficacy to trigger vesicle fusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Augustine, G.J. & Neher, E. Calcium requirements for secretion in bovine chromaffin cells. J. Physiol. 450, 247–271 (1992).
Monck, J.R., Robinson, I.M., Escobar, A.L., Vergara, J.L. & Fernandez, J.M. Pulsed laser Imaging of rapid Ca2+ gradients in excitable cells. Biophys. J. 67, 505–514 (1994).
Melamed-Book, N., Kachalsky, S.G., Kaiserman, I. & Rhamimoff, R. Neuronal calcium sparks and intracellular calcium “noise”. Proc. Natl. Acad. Sci. USA 96, 15217–15221 (1999).
Chad, J.E. & Eckert, R. Calcium domains associated with individual channels can account for anomalous voltage relations of Ca-dependent responses. Biophys. J. 45, 993–999 (1984).
Simon, S.M. & Llinás, R.R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys. J. 48, 485–498 (1985).
Sala, F. & Hernández-Cruz, A. Calcium diffusion modeling in a spherical neuron: relevance of buffering properties. Biophys. J. 57, 312–324 (1990).
Nowycky, M.C. & Pinter, M.J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys. J. 64, 77–91 (1993).
Naraghi, M. & Neher, E. Linearized buffered Ca2+ diffusion in microdomains and Its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 17, 6961–6973 (1997).
Augustine, G.J. & Neher, E. Neuronal Ca2+ signalling takes the local route. Curr. Opin. Neurobiol. 2, 302–307 (1992).
Neher, E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron 20, 389–399 (1998).
Llinás, R., Sugimori, M. & Silver, R.B. Microdomains of high calcium concentration in a presynaptic terminal. Science 256, 677–679 (1992).
Parnas, I. & Parnas, H. Different mechanisms control the amount and time course of neurotransmitter release. J. Physiol. (Lond.) 517, 629 (1999).
Horrigan, F.T. & Bookman, R.J. Releasable pools and the kinetics of exocytosis in chromaffin cells. Neuron 13, 1119–1129 (1994).
Barg, S. et al. Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic beta cells. Biophys. J. 81, 3308–3323 (2001).
Khanna, B., Tabares, L., Alvarez de Toledo, G. & Lindau, M. Action potentials and quantal release in chromaffin cells. Biophys. J. 82, 10–11 (2002).
Etter, E.F., Kuhn, M.A. & Fay, F.S. Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator. J. Biol. Chem. 269, 10141–10149 (1994).
Tucker, T. & Tettiplace, R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron 15, 1323–1335 (1995).
Omann, G.M. & Axelrod, D. Membrane-proximal calicum transients in stimulated neutrophils detected by total internal reflection fluorescence. Biophys. J. 71, 2885–2891 (1996).
Cleeman, L., DiMassa, G. & Morad, M. Ca2+ sparks within 200 nm of the sarcolemma of rat ventricular cells: evidence from total internal reflection fluorescence microscopy. Advan. Exp. Med. Biol. 430, 57–65 (1997).
Zenisek, D., Davila, V., Wan, L. & Almers, W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J. Neurosci. 23, 2538–2548 (2003).
Chow, R.H., Klingauf, J. & Neher, E. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proc. Natl. Acad. Sci. USA 91, 12765–12769 (1994).
Steyer, J.A. & Almers, W. Tracking single secretory granules in live chromaffin cells by evanescent-field fluorescence microscopy. Biophys. J. 76, 2262–2271 (1999).
Oheim, M. & Stühmer, W. Tracking individual granules through the actin cortex. Eur. Biophys. J. 29, 67–89 (2000).
Johns, L.M., Levitan, E.S., Shelden, E.A., Holz, R.W. & Axelrod, D. Restriction of secretory granule motion near the plasma membrane of chromaffin cells. J. Cell Biol. 153, 177–190 (2001).
Klingauf, J. & Neher, E. Modeling buffered Ca2+ diffusion near the membrane: Implications for secretion in neuroendocrine cells. Biophys. J. 72, 674–690 (1997).
Adler, E.M., Augustine, G.J., Duffy, S.N. & Charlton, M.P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J. Neurosci. 11, 1496–1507 (1991).
Roberts, W.M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J. Neurosci. 14, 3246–3262 (1994).
Gosh, R.N. & Webb, W.W. Automated detection and tracking of individual and clustered cell surface low density lipoprotein receptor molecules. Biophys. J. 66, 1301–1318 (1994).
Augustine, G.J. How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 11, 320–326 (2001).
Rettig, J. & Neher, E. Emerging roles of presynaptic proteins in Ca2+ triggered exocytosis. Science 298, 781–785 (2002).
Atlas, D. Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism. J. Neurochem. 77, 972–985 (2001).
Schroeder, T.J., Jankowski, J.A., Senyshyn, J. & Holz, R.W. Zones of exocytotic release on bovine adrenal medullary cells in culture. J. Biol. Chem. 269, 17215–17220 (1994).
Robinson, I.M., Finnegan, J.M., Monck, J.R., Wightman, R.M. & Fernandez, J.M. Colocalization of calcium entry and exocytotic release sites in adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA 92, 2474–2478 (1995).
Wick, P.F., Trenkle, J.M. & Holz, R.W. Punctate appearance of dopamine-beta-hydroxylase on the chromaffin cell-surface reflects the fusion of individual chromaffin granules upon exocytosis. J. Neurosci. 80, 847–860 (1997).
Oheim, M., Loerke, D., Stühmer, W. & Chow, R.H. Multiple stimulation-dependent processes regulate the size of the releasable pool of vesicles. Eur. Biophys. J. 28, 91–101 (1999).
Voets, T., Neher, E. & Moser, T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron 23, 607–615 (1999).
Bittner, M.A. & Holz, R.W. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J. Biol. Chem. 267, 16219–16225 (1992).
Rüden, L.v. & Neher, E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science 262, 1061–1065 (1993).
Smith, C., Moser, T., Xu, T. & Neher, E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron 20, 1243–1253 (1998).
Stevens, C.F. & Wesseling, J.F. Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron 21, 415–424 (1998).
Gomis, A., Burrone, J. & Lagnodo, L. Two actions of calcium regulate the supply of releasable vesicles at the ribbon synapse of retinal bipolar cells. J. Neurosci. 19, 6309–6317 (1999).
Voets, T. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron 28, 537–545 (2000).
Ashery, U. et al. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. EMBO J. 19, 3586–3596 (2000).
Lev-Ram. Calcium transients in in cerebellar Prukinje neurones evoked by intracellular stimulation. J. Neurophysiol. 68, 1167–1177 (1992).
Cheng, H. et al. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys. J. 76, 606–617 (1999).
Zenisek, D., Steyer, J.A., Feldman, M.E. & Almers, W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron 35, 1085–1097 (2002).
Wightman, R.M. et al. Temporarily resolved catecholamine spikes correspond to single vesicle release from individual chromaffin granules. Proc. Natl. Acad. Sci. USA 88, 10754–10758 (1991).
Chow, R.H., Rüden, L.v. & Neher, E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature 356, 60–63 (1992).
Schulte, A. & Chow, R.H. Cylindrically etched carbon-fiber microelectrodes for low-noise amperometric recording. Anal. Chem. 70, 985–990 (1998).
Acknowledgements
We thank J. B. Sørensen for chromaffin cells and carbon-fiber electrodes, B. Barbour, K. Delaney, E. Neher and members of our labs for comments on the manuscript. Supported by the Ministère de la Recherche (ACI 'young investigator group' 5242 to M.O.), the Max-Planck Society and SFB 406 (to W.S. and T.M.) and the Alexander-von-Humboldt Foundation (to U.B. and M.O.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Becherer, U., Moser, T., Stühmer, W. et al. Calcium regulates exocytosis at the level of single vesicles. Nat Neurosci 6, 846–853 (2003). https://doi.org/10.1038/nn1087
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1087
This article is cited by
-
How does the stimulus define exocytosis in adrenal chromaffin cells?
Pflügers Archiv - European Journal of Physiology (2018)
-
The role of F-actin in the transport and secretion of chromaffin granules: an historic perspective
Pflügers Archiv - European Journal of Physiology (2018)
-
A Model for the Acrosome Reaction in Mammalian Sperm
Bulletin of Mathematical Biology (2018)
-
F-Actin–Myosin II Inhibitors Affect Chromaffin Granule Plasma Membrane Distance and Fusion Kinetics by Retraction of the Cytoskeletal Cortex
Journal of Molecular Neuroscience (2012)
-
From the stochasticity of molecular processes to the variability of synaptic transmission
Nature Reviews Neuroscience (2011)