Abstract
Outgrowth of axons in the central nervous system is governed by specific molecular cues. Molecules detected so far act as ligands that bind to specific receptors. Here, we report a new membrane-associated lipid phosphate phosphatase that we have named plasticity-related gene 1 (PRG-1), which facilitates axonal outgrowth during development and regenerative sprouting. PRG-1 is specifically expressed in neurons and is located in the membranes of outgrowing axons. There, it acts as an ecto-enzyme and attenuates phospholipid-induced axon collapse in neurons and facilitates outgrowth in the hippocampus. Thus, we propose a novel mechanism by which axons are able to control phospholipid-mediated signaling and overcome the growth-inhibiting, phospholipid-rich environment of the extracellular space.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tessier-Lavigne, M. & Goodman, C.S. The molecular biology of axon guidance. Science 274, 1123–1133 (1996).
Goodman, C.S. Mechanisms and molecules that control growth cone guidance. Annu. Rev. Neurosci. 19, 341–377 (1996).
Super, H. & Soriano, E. The organization of the embryonic and early postnatal murine hippocampus. II. Development of entorhinal, commissural, and septal connections studied with the lipophilic tracer DiI. J. Comp. Neurol. 344, 101–120 (1994).
Cotman, C., Gentry, C. & Steward, O. Synaptic replacement in the dentate gyrus after unilateral entorhinal lesion: electron microscopic analysis of the extent of replacement of synapses by the remaining entorhinal cortex. J. Neurocytol. 6, 455–464 (1977).
Frotscher, M., Heimrich, B. & Deller, T. Sprouting in the hippocampus is layer- specific. Trends Neurosci. 20, 218–223 (1997).
Stein, E. & Tessier-Lavigne, M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291, 1928–1938 (2001).
Colamarino, S.A. & Tessier-Lavigne, M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell 81, 621–629 (1995).
Kobayashi, H., Koppel, A.M., Luo, Y. & Raper, J.A. A role for collapsin-1 in olfactory and cranial sensory axon guidance. J. Neurosci. 17, 8339–8352 (1997).
Stein, E. et al. A role for the Eph ligand ephrin-A3 in entorhino-hippocampal axon targeting. J. Neurosci. 19, 8885–8893 (1999).
Steup, A. et al. Sema3C and netrin-1 differentially affect axon growth in the hippocampal formation. Mol. Cell. Neurosci. 15, 141–155 (2000).
Jalink, K. et al. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 126, 801–810 (1994).
Tigyi, G. et al. Lysophosphatidic acid-induced neurite retraction in PC12 cells: neurite-protective effects of cyclic AMP signaling. J. Neurochem. 66, 549–558 (1996).
Moolenaar, W.H. Development of our current understanding of bioactive lysophospholipids. Ann. NY Acad. Sci. 905, 1–10 (2000).
Skutella, T. & Nitsch, R. New molecules for hippocampal development. Trends Neurosci. 24, 107–113 (2001).
Savaskan, N.E. & Nitsch, R. Molecules involved in reactive sprouting in the hippocampus. Rev. Neurosci. 12, 195–215 (2001).
Jalink, K., Hordijk, P.L. & Moolenaar, W.H. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim. Biophys. Acta 1198, 185–196 (1994).
Moolenaar, W.H. Lysophosphatidic acid signalling. Curr. Opin. Cell Biol. 7, 203–210 (1995).
Zhang, N., Zhang, J., Purcell, K.J., Cheng, Y. & Howard, K. The Drosophila protein Wunen repels migrating germ cells. Nature 385, 64–67 (1997).
Fukushima, N., Weiner, J.A. & Chun, J. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev. Biol. 228, 6–18 (2000).
Neuwald, A.F. An unexpected structural relationship between integral membrane phosphatases and soluble haloperoxidases. Protein Sci. 6, 1764–1767 (1997).
Doren, M.V. & Lehmann, R. Cell migration: don't tread on me. Curr. Biol. 7, R148–R150 (1997).
Bräuer, A.U. et al. Molecular and functional analysis of hyperpolarization- activated pacemaker channels in the hippocampus after entorhinal cortex lesion. FASEB J. 15, 2689–2701 (2001).
Bayer, S.A., Yackel, J.W. & Puri, P.S. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science 216, 890–892 (1982).
Deller, T., Frotscher, M. & Nitsch, R. Morphological evidence for the sprouting of inhibitory commissural fibers in response to the lesion of the excitatory entorhinal input to the rat dentate gyrus. J. Neurosci. 15, 6868–6878 (1995).
Jalink, K., Eichholtz, T., Postma, F.R., van Corven, E.J. & Moolenaar, W.H. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 4, 247–255 (1993).
Bothmer, J., Markerink, M. & Jolles, J. Brain phosphatidic acid and polyphosphoinositide formation in a broken cell preparation: regional distribution and the effect of age. Neurochem. Int. 21, 223–228 (1992).
Moolenaar, W.H., Kranenburg, O., Postma, F.R. & Zondag, G.C. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr. Opin. Cell Biol. 9, 168–173 (1997).
Brindley, D.N. & Waggoner, D.W. Mammalian lipid phosphate phosphohydrolases. J. Biol. Chem. 273, 24281–24284 (1998).
Kanoh, H., Kai, M. & Wada, I. Phosphatidic acid phosphatase from mammalian tissues: discovery of channel-like proteins with unexpected functions. Biochim. Biophys. Acta 1348, 56–62 (1997).
Hla, T., Lee, M.J., Ancellin, N., Paik, J.H. & Kluk, M.J. Lysophospholipids—receptor revelations. Science 294, 1875–1878 (2001).
Bräuer, A.U., Savaskan, N.E., Plaschke, M., Ninnemann, O. & Nitsch, R. Perforant path lesion induces up-regulation of stathmin messenger RNA, but not SCG10 messenger RNA, in the adult rat hippocampus. Neuroscience 102, 515–526 (2001).
Meier, S. et al. Molecular analysis of Nogo genes in the hippocampus during develoment and following lesion and seizure. FASEB J. (Apr. 8, 2003).
Savaskan, N.E. et al. Outgrowth-promoting molecules in the adult hippocampus after perforant path lesion. Eur. J. Neurosci. 12, 1024–1032 (2000).
Savaskan, N.E. et al. Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J. 17, 112–114 (2003).
Imai, A., Furui, T., Tamaya, T. & Mills, G.B. A gonadotropin-releasing hormone-responsive phosphatase hydrolyses lysophosphatidic acid within the plasma membrane of ovarian cancer cells. J. Clin. Endocrinol. Metab. 85, 3370–3375 (2000).
Hooks, S.B. et al. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 276, 4611–4621 (2001).
Bräuer, A.U. et al. IG-molecule Kilon shows differential expression pattern from LAMP in the developing and adult rat hippocampus. Hippocampus 10, 632–644 (2000).
Acknowledgements
The authors thank T. Jöns for help throughout the course of antibody generation; D. Lajko, P. Thiele, M. Petzold, B. Brokowski, E. Bürger and G. Duwe for technical support; S. Lewandowski and D. Wachenschwanz for help with photography and graphic design; K. Rosegger for editorial assistance; and G. Wulczyn, F. Zipp and B. Heimrich for critical remarks on the manuscript. This study was supported by the Deutsche Forschungsgemeinschaft (DFG) SFB 515/A5. N.E.S. is an Investigator of the Charité Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1.
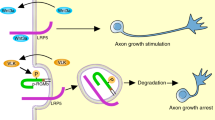
Schematic diagram of the proposed axon growth mechanisms in a phospholipid-enriched environment. Axons that are sensitive to a repulsive phospholipid (in this case LPA) but do not express PRG-1 are unable to cross a phospholipid-rich barrier. In contrast, PRG-1-expressing neurons can grow through a phospholipid-rich zone by locally depleting the extracellular pool of repulsive phospholipids acting as ligands on EDG receptors. In this way, PRG-1 may regulate the activation of EDG receptors and thereby modulate axonal outgrowth. Further on, it is hypothesized that other phospholipids than LPA also induce rapid axon collapse and that PRG-1 might be involved in this signaling pathway. (GIF 33 kb)
Rights and permissions
About this article
Cite this article
Bräuer, A., Savaskan, N., Kühn, H. et al. A new phospholipid phosphatase, PRG-1, is involved in axon growth and regenerative sprouting. Nat Neurosci 6, 572–578 (2003). https://doi.org/10.1038/nn1052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1052
This article is cited by
-
Integrated analyses reveal evolutionarily conserved and specific injury response genes in dorsal root ganglion
Scientific Data (2022)
-
Lysophosphatidic Acid Signalling in Nervous System Development and Function
NeuroMolecular Medicine (2021)
-
Synaptic phospholipids as a new target for cortical hyperexcitability and E/I balance in psychiatric disorders
Molecular Psychiatry (2018)
-
Genome-Wide Array Analysis Reveals Novel Genomic Regions and Candidate Gene for Intellectual Disability
Molecular Diagnosis & Therapy (2018)
-
Dynamics of the mouse brain cortical synaptic proteome during postnatal brain development
Scientific Reports (2016)