Abstract
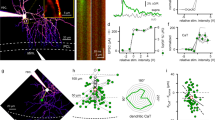
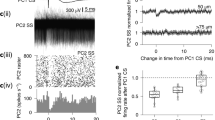
A single neurotransmitter elicits diverse physiological responses through activation of multiple receptor subtypes and/or heterosynaptic interactions involving distinct synaptic targets. We found that a typical excitatory transmitter released from the climbing fiber (CF) in the cerebellar cortex not only excited Purkinje cells directly but also presynaptically inhibited GABAergic transmission from interneurons converging on the same Purkinje cells. Both homosynaptic and heterosynaptic actions of the CF transmitter (possibly glutamate) were mediated by activation of AMPA receptors. Dual AMPA receptor-mediated functions of excitation and disinhibition may ensure transmission of cerebellar CF signals controlling sensorimotor coordination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Byrne, J. H. & Kandel, E. R. Presynaptic facilitation revisited: state and time dependence. J. Neurosci. 16, 425–435 (1996).
Dixon, D. & Atwood, H. L. Conjoint action of phosphatidylinositol and adenylate cyclase systems in serotonin-induced facilitation at the crayfish neuromuscular junction. J. Neurophysiol. 62, 1251–1259 (1989).
Goy, M. F. & Kravitz, E. A. Cyclic AMP only partially mediates the actions of serotonin at lobster neuromuscular junctions. J. Neurosci. 9, 369–379 (1989).
Huang, Y.-Y. & Kandel, E. R. Modulation of both the early and the late phase of mossy fiber LTP by the activation of β-adrenergic receptors. Neuron 16, 611–617 (1996).
Mitoma, H. & Konishi, S. Long-lasting facilitation of inhibitory transmission by monoaminergic and cAMP-dependent mechanism in rat cerebellar GABAergic synapses. Neurosci. Lett. 217, 141–144 (1996).
Rodríguez-Moreno, A., Herreras, O. & Lerma, J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19, 893–901 (1997).
Min, M.-Y., Melyan, Z. & Kullmann, D. M. Synaptically released glutamate reduces γ-aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors. Proc. Natl. Acad. Sci. USA 96, 9932–9937 (1999).
Brickley, S. G., Cull-Candy, S. G. & Farrant, M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. (Lond.) 497, 753–759 (1996).
Rossi, D. J. & Hamann, M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron 20, 783–795 (1998).
Konnerth, A., Llano, I. & Armstrong, C. M. Synaptic currents in cerebellar Purkinje cells. Proc. Natl. Acad. Sci. USA 87, 2662–2665 (1990).
Vincent, P., Armstrong, C. M. & Marty, A. Inhibitory synaptic currents in rat cerebellar Purkinje cells: modulation by postsynaptic depolarization. J. Physiol. (Lond.) 456, 453–471 (1992).
Mitoma, H. & Konishi, S. Monoaminergic long-term facilitation of GABA-mediated inhibitory transmission at cerebellar synapses. Neuroscience 88, 871–883 (1999).
Konishi, S., Mitoma, H., Ishida, M. & Shinozaki, H. Cerebellar GABAergic transmission reciprocally regulated by monoaminergic receptor-operated facilitation and metabotropic glutamate receptor-operated inhibition. Pharmacol. Rev. Commun. 8, 169–172 (1996).
Bekkers, J. M. & Stevens, C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature 346, 724–729 (1990).
Clements, J. D. A. A statistical test for demonstrating a presynaptic site of action for a modulator of synaptic amplitude. J. Neurosci. Methods 31, 75–88 (1990).
Korn, H. & Faber, D. S. Quantal analysis and synaptic efficacy in the CNS. Trends Neurosci. 14, 439–445 (1991).
Vollenweider, F. X., Cuenod, M. & Do, K. Q. Effect of climbing fiber deprivation on release of endogenous aspartate, glutamate, and homocysteate in slices of rat cerebellar hemispheres and vermis. J. Neurochem. 54, 1533–1540 (1990).
Conn, P. J. & Pin, J.-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237 (1997).
Glitsch, M., Llano, I. & Marty, A. Glutamate as a candidate retrograde messenger at interneurone-Purkinje cell synapses of rat cerebellum. J. Physiol. (Lond.) 497, 531–537 (1996).
Konishi, S. & Mitoma, H. in The Role of Adenosine in the Nervous System (ed. Okada, Y.) 89–95 (Elsevier, New York, 1997).
Bleakman, D. & Lodge, D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology 37, 1187–1204 (1998).
Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61 (1999).
Lerma, J., Morales, M., Vicente, M. A. & Herreras, O. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci. 20, 9–12 (1997).
Clarke, V. R. J. et al. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389, 599–603 (1997).
Vignes, M. & Collingridge, G. L. The synaptic activation of kainate receptors. Nature 388, 179–182 (1997).
Castillo, P. E., Malenka, R. C. & Nicoll, R. A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186 (1997).
Partin, K. M. et al. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11, 1069–1082 (1993).
Yamada, K. A. & Tang, C.-M. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J. Neurosci. 13, 3904–3915 (1993).
Rodríguez-Moreno, A. & Lerma, J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20, 1211–1218 (1998).
Jan, L. Y. & Jan, Y. N. Peptidergic transmission in sympathetic ganglia of the frog. J. Physiol. (Lond.) 327, 219–246 (1982).
Min, M.-Y., Rusakov, D. A. & Kullmann, D. M. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: role of glutamate diffusion. Neuron 21, 561–570 (1998).
Kano, M., Rexhausen, U., Dreessen, J. & Konnerth, A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory signals in cerebellar Purkinje cells. Nature 356, 601–604 (1992).
Wang, Y., Small, D. L., Stanimirovic, D. B., Morley, P. & Durkin, J. P. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature 389, 502–504 (1997).
Keinänen, K. et al. A family of AMPA-selective glutamate receptors. Science 249, 556–560 (1990).
Petralia, R. S. & Wenthold, R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J. Comp. Neurol. 318, 329–354 (1992).
Mitoma, H., Kobayashi, T., Song, S.-Y. & Konishi, S. Enhancement by serotonin of GABA-mediated inhibitory synaptic currents in rat cerebellar Purkinje cells. Neurosci. Lett. 173, 127–130 (1994).
Kondo, S. & Marty, A. Protein kinase A-mediated enhancement of minature frequency by noradrenaline in rat cerebellar stellate cells. J. Physiol. (Lond.) 498, 165–176 (1997).
Ito, M. Long-term depression. Annu. Rev. Neurosci. 12, 85–102 (1989).
Jacobs, B. L. & Fornal, C. A. 5-HT and motor control: a hypothesis. Trends Neurosci. 16, 346–352 (1993).
Mauk, M. D. Roles of cerebellar cortex and nuclei in motor learning: contradictions or clues? Neuron 18, 343–346 (1997).
Acknowledgements
We thank N. Bowery, T. Murakoshi, H. Suzuki and K. Yoshioka for comments on the manuscript and D. Lodge at Eli Lilly and Fujisawa Pharmaceutical for the gifts of GYKI 53655 and FK506, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satake, S., Saitow, F., Yamada, J. et al. Synaptic activation of AMPA receptors inhibits GABA release from cerebellar interneurons. Nat Neurosci 3, 551–558 (2000). https://doi.org/10.1038/75718
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/75718
This article is cited by
-
Afferent convergence to a shared population of interneuron AMPA receptors
Nature Communications (2023)
-
mGluR2/3 agonist LY379268 rescues NMDA and GABAA receptor level deficits induced in a two-hit mouse model of schizophrenia
Psychopharmacology (2016)
-
Distributed synergistic plasticity and cerebellar learning
Nature Reviews Neuroscience (2012)
-
Presynaptic glutamate receptors: physiological functions and mechanisms of action
Nature Reviews Neuroscience (2008)