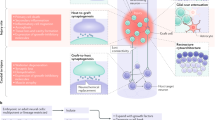
Abstract
Spinal cord injury can lead to severe motor, sensory and autonomic dysfunction. Currently, there is no effective treatment for the injured spinal cord. The transplantation of Schwann cells, neural stem cells or progenitor cells, olfactory ensheathing cells, oligodendrocyte precursor cells and mesenchymal stem cells has been investigated as potential therapies for spinal cord injury. However, little is known about the mechanisms through which these individual cell types promote repair and functional improvements. The five most commonly proposed mechanisms include neuroprotection, immunomodulation, axon regeneration, neuronal relay formation and myelin regeneration. A better understanding of the mechanisms whereby these cells promote functional improvements, as well as an appreciation of the obstacles in implementing these therapies and effectively modeling spinal cord injury, will be important to make cell transplantation a viable clinical option and may lead to the development of more targeted therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lee, B.B., Cripps, R.A., Fitzharris, M. & Wing, P.C. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 52, 110–116 (2014).
Mackay-Sim, A. & St John, J.A. Olfactory ensheathing cells from the nose: clinical application in human spinal cord injuries. Exp. Neurol. 229, 174–180 (2011).
Saberi, H. et al. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J. Neurosurg. Spine 15, 515–525 (2011).
Ramón y Cajal, S., DeFelipe, J. & Jones, E.G. Cajal's Degeneration and Regeneration of the Nervous System (Oxford Univ. Press, 1991).
David, S. & Aguayo, A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214, 931–933 (1981).
Bunge, M.B. Efficacy of Schwann Cell (SC) transplantation for spinal cord repair is improved with combinatorial strategies. J. Physiol. (Lond.) 594, 3533–3538 (2016).
Das, G.D. Neural transplantation: an historical perspective. Neurosci. Biobehav. Rev. 14, 389–401 (1990).
Björklund, A. & Lindvall, O. Cell replacement therapies for central nervous system disorders. Nat. Neurosci. 3, 537–544 (2000).
Bregman, B.S. et al. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp. Neurol. 123, 3–16 (1993).
Wirth, E.D. III et al. Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J. Neurotrauma 18, 911–929 (2001).
Tetzlaff, W. et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 28, 1611–1682 (2011).
Lu, P. et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83, 789–796 (2014).
Yang, N. et al. Generation of oligodendroglial cells by direct lineage conversion. Nat. Biotechnol. 31, 434–439 (2013).
Piltti, K.M., Salazar, D.L., Uchida, N., Cummings, B.J. & Anderson, A.J. Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl. Med. 2, 204–216 (2013).
Kwon, B.K., Tetzlaff, W., Grauer, J.N., Beiner, J. & Vaccaro, A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 4, 451–464 (2004).
Norenberg, M.D., Smith, J. & Marcillo, A. The pathology of human spinal cord injury: defining the problems. J. Neurotrauma 21, 429–440 (2004).
Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol. Exp. (Warsz.) 71, 281–299 (2011).
Raisman, G. Olfactory ensheathing cells - another miracle cure for spinal cord injury? Nat. Rev. Neurosci. 2, 369–375 (2001).
Plemel, J.R. et al. A graded forceps crush spinal cord injury model in mice. J. Neurotrauma 25, 350–370 (2008).
Basso, D.M., Beattie, M.S. & Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 (1996).
Schucht, P., Raineteau, O., Schwab, M.E. & Fouad, K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176, 143–153 (2002).
Kakulas, B.A. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord 42, 549–563 (2004).
Rabchevsky, A.G., Fugaccia, I., Sullivan, P.G., Blades, D.A. & Scheff, S.W. Efficacy of methylprednisolone therapy for the injured rat spinal cord. J. Neurosci. Res. 68, 7–18 (2002).
Kwon, B.K. et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma 28, 1545–1588 (2011).
Pearse, D.D. et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 10, 610–616 (2004).
Biernaskie, J. et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J. Neurosci. 27, 9545–9559 (2007).
Barbour, H.R., Plant, C.D., Harvey, A.R. & Plant, G.W. Tissue sparing, behavioral recovery, supraspinal axonal sparing/regeneration following sub-acute glial transplantation in a model of spinal cord contusion. BMC Neurosci. 14, 106 (2013).
Crowe, M.J., Bresnahan, J.C., Shuman, S.L., Masters, J.N. & Beattie, M.S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 3, 73–76 (1997).
Lankford, K.L., Imaizumi, T., Honmou, O. & Kocsis, J.D. A quantitative morphometric analysis of rat spinal cord remyelination following transplantation of allogenic Schwann cells. J. Comp. Neurol. 443, 259–274 (2002).
Hawryluk, G.W. et al. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 21, 2222–2238 (2012).
Cantinieaux, D. et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One 8, e69515 (2013).
Sharp, J., Frame, J., Siegenthaler, M., Nistor, G. & Keirstead, H.S. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 28, 152–163 (2010).
Gu, W.D. et al. Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology 30, 205–217 (2010).
Zhang, S.X., Huang, F., Gates, M. & Holmberg, E.G. Role of endogenous Schwann cells in tissue repair after spinal cord injury. Neural Regen. Res. 8, 177–185 (2013).
Ritfeld, G.J. et al. The role of brain-derived neurotrophic factor in bone marrow stromal cell-mediated spinal cord repair. Cell Transplant. 24, 2209–2220 (2015).
Tator, C.H. & Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75, 15–26 (1991).
Okon, E.B. et al. Intraparenchymal microdialysis after acute spinal cord injury reveals differential metabolic responses to contusive versus compressive mechanisms of injury. J. Neurotrauma 30, 1564–1576 (2013).
Figley, S.A., Khosravi, R., Legasto, J.M., Tseng, Y.F. & Fehlings, M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma 31, 541–552 (2014).
Stokes, B.T. & Reier, P.J. Oxygen transport in intraspinal fetal grafts: graft-host relations. Exp. Neurol. 111, 312–323 (1991).
Horner, P.J., Reier, P.J. & Stokes, B.T. Quantitative analysis of vascularization and cytochrome oxidase following fetal transplantation in the contused rat spinal cord. J. Comp. Neurol. 364, 690–703 (1996).
Horner, P.J. & Stokes, B.T. Fetal transplantation following spinal contusion injury results in chronic alterations in CNS glucose metabolism. Exp. Neurol. 133, 231–243 (1995).
López-Vales, R., Garcia-Alias, G., Fores, J., Navarro, X. & Verdu, E. Increased expression of cyclo-oxygenase 2 and vascular endothelial growth factor in lesioned spinal cord by transplanted olfactory ensheathing cells. J. Neurotrauma 21, 1031–1043 (2004).
Richter, M.W., Fletcher, P.A., Liu, J., Tetzlaff, W. & Roskams, A.J. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J. Neurosci. 25, 10700–10711 (2005).
Ramer, L.M. et al. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J. Comp. Neurol. 473, 1–15 (2004).
Quaranta, M., Borisov, S.M. & Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 4, 115–157 (2012).
Bittner, C.X. et al. High resolution measurement of the glycolytic rate. Front. Neuroenergetics https://doi.org/10.3389/fnene.2010.00026 (2010).
Plemel, J.R., Yong, V.W. & Stirling, D.P. Immune modulatory therapies for spinal cord injury - past, present and future. Exp. Neurol. 258, 91–104 (2014).
Gadani, S.P., Walsh, J.T., Lukens, J.R. & Kipnis, J. Dealing with danger in the CNS: the response of the immune system to injury. Neuron 87, 47–62 (2015).
Jones, T.B., McDaniel, E.E. & Popovich, P.G. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr. Pharm. Des. 11, 1223–1236 (2005).
Gensel, J.C. & Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619, 1–11 (2015).
Kigerl, K.A. et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 (2009).
Miron, V.E. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013).
Xue, J. et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40, 274–288 (2014).
Cusimano, M. et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 135, 447–460 (2012).
Nakajima, H. et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 29, 1614–1625 (2012).
Abrams, M.B. et al. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor. Neurol. Neurosci. 27, 307–321 (2009).
DePaul, M.A. et al. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci. Rep. 5, 16795 (2015).
Filous, A.R. & Silver, J. Targeting astrocytes in CNS injury and disease: A translational research approach. Prog. Neurobiol. 144, 173–187 (2016).
Schwab, M.E. & Strittmatter, S.M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 27, 53–60 (2014).
He, Z. & Jin, Y. Intrinsic control of axon regeneration. Neuron 90, 437–451 (2016).
Xu, X.M., Guenard, V., Kleitman, N. & Bunge, M.B. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult-rat spinal-cord. J. Comp. Neurol. 351, 145–160 (1995).
Takeoka, A. et al. Axon regeneration can facilitate or suppress hindlimb function after olfactory ensheathing glia transplantation. J. Neurosci. 31, 4298–4310 (2011).OEC transplantation promotes axon regeneration that can suppress hindlimb motor function. This study articulates the need to understand how regenerated axons can be directed towards forming circuitry that underlies functional improvements.
Lu, P. et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 (2012).This study demonstrates relay formation between descending motor pathways and transplant-derived neurons that project axons long distances in the injured adult mammalian spinal cord.
Lu, P., Yang, H., Jones, L.L., Filbin, M.T. & Tuszynski, M.H. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 24, 6402–6409 (2004).
Fouad, K. et al. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J. Neurosci. 25, 1169–1178 (2005).Combinatorial therapy of Schwann Cells, OECs and chABC promotes hindlimb motor function following complete transection of the spinal cord that likely relies on serotonergic axon regeneration caudal to the injury site.
Krishna, V. et al. Biomaterial-based interventions for neuronal regeneration and functional recovery in rodent model of spinal cord injury: a systematic review. J. Spinal Cord Med. 36, 174–190 (2013).
Xu, X.M., Chen, A., Guenard, V., Kleitman, N. & Bunge, M.B. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 26, 1–16 (1997).
Kadoya, K. et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 22, 479–487 (2016)This paper describes robust corticospinal regeneration and synapse formation caudal to injury following neural graft transplantation. Regenerating corticospinal axons long distances represents a key goal for the field, given the pathway's importance in voluntary movement.
Lemon, R.N. Descending pathways in motor control. Annu. Rev. Neurosci. 31, 195–218 (2008).
Tuszynski, M.H. & Steward, O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron 74, 777–791 (2012).
Lu, P. et al. Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 32, 8208–8218 (2012).
Geoffroy, C.G., Hilton, B.J., Tetzlaff, W. & Zheng, B. Evidence for an age-dependent decline in axon regeneration in the adult mammalian central nervous system. Cell Rep. 15, 238–246 (2016).This paper demonstrates an age-related decline in CNS axon regeneration. Whether axon growth following cell transplantation after SCI is influenced by aging is unknown and is an important avenue for further research.
Williams, R.R., Henao, M., Pearse, D.D. & Bunge, M.B. Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant. 24, 115–131 (2015).
Burda, J.E. & Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248 (2014).
Cregg, J.M. et al. Functional regeneration beyond the glial scar. Exp. Neurol. 253, 197–207 (2014).
Anderson, M.A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Crigler, L., Robey, R.C., Asawachaicharn, A., Gaupp, D. & Phinney, D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 198, 54–64 (2006).
Weidner, N., Ner, A., Salimi, N. & Tuszynski, M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA 98, 3513–3518 (2001).
Hilton, B.J. et al. Dorsolateral funiculus lesioning of the mouse cervical spinal cord at C4 but not at C6 results in sustained forelimb motor deficits. J. Neurotrauma 30, 1070–1083 (2013).
Ertürk, A. & Bradke, F. High-resolution imaging of entire organs by 3-dimensional imaging of solvent cleared organs (3DISCO). Exp. Neurol. 242, 57–64 (2013).
Wang, Z.M., Reynolds, A., Kirry, A., Nienhaus, C. & Blackmore, M.G. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 35, 3139–3145 (2015).
Fouad, K. & Tetzlaff, W. Rehabilitative training and plasticity following spinal cord injury. Exp. Neurol. 235, 91–99 (2012).
Hilton, B.J. et al. Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J. Neurosci. 36, 4080–4092 (2016).This paper provides the first demonstration that a spared dorsolateral corticospinal pathway underlies recovery following spinal cord injury. Similar approaches can be used to assess circuit functionality in the context of cell transplantation.
Roth, B.L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Reier, P.J. Neural tissue grafts and repair of the injured spinal cord. Neuropathol. Appl. Neurobiol. 11, 81–104 (1985).
Bregman, B.S. & Reier, P.J. Neural tissue transplants rescue axotomized rubrospinal cells from retrograde death. J. Comp. Neurol. 244, 86–95 (1986).
Jakeman, L.B. & Reier, P.J. Axonal projections between fetal spinal-cord transplants and the adult-rat spinal-cord - a neuroanatomical tracing study of local interactions. J. Comp. Neurol. 307, 311–334 (1991).
Wictorin, K., Brundin, P., Gustavii, B., Lindvall, O. & Bjorklund, A. Reformation of long axon pathways in adult rat central nervous system by human forebrain neuroblasts. Nature 347, 556–558 (1990).
Steward, O., Sharp, K.G., Yee, K.M., Hatch, M.N. & Bonner, J.F. Characterization of ectopic colonies that form in widespread areas of the nervous system with neural stem cell transplants into the site of a severe spinal cord injury. J. Neurosci. 34, 14013–14021 (2014).
Sontag, C.J., Uchida, N., Cummings, B.J. & Anderson, A.J. Injury to the spinal cord niche alters the engraftment dynamics of human neural stem cells. Stem Cell Rep. 2, 620–632 (2014).
Gold, M.S. & Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257 (2010).
Lasiene, J., Shupe, L., Perlmutter, S. & Horner, P. No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J. Neurosci. 28, 3887–3896 (2008).Tracing rubrospinal axons reveals little evidence of chronic demyelination after contusive SCI, but shorter internodes are observed in many axons, suggestive of extensive remyelination.
Totoiu, M.O. & Keirstead, H.S. Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol. 486, 373–383 (2005).
Emery, E. et al. Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 89, 911–920 (1998).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Funfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Blight, A.R. & Young, W. Central axons in injured cat spinal-cord recover electrophysiological function following remyelination by Schwann-cells. J. Neurol. Sci. 91, 15–34 (1989).
Smith, K.J., Blakemore, W.F. & Mcdonald, W.I. Central remyelination restores secure conduction. Nature 280, 395–396 (1979).
Myers, S.A., Bankston, A.N., Burke, D.A., Ohri, S.S. & Whittemore, S.R. Does the preclinical evidence for functional remyelination following myelinating cell engraftment into the injured spinal cord support progression to clinical trials? Exp. Neurol. 183B, 560–572 (2016).
Plemel, J.R. et al. Remyelination after spinal cord injury: is it a target for repair? Prog. Neurobiol. 117, 54–72 (2014).
Takami, T. et al. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 22, 6670–6681 (2002).
Sparling, J.S. et al. Schwann cells generated from neonatal skin-derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J. Neurosci. 35, 6714–6730 (2015).
Wiliams, R.R. & Bunge, M.B. Schwann cell transplantation: a repair strategy for spinal cord injury? Prog. Brain Res. 201, 295–312 (2012).
Hill, C.E., Moon, L.D., Wood, P.M. & Bunge, M.B. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia 53, 338–343 (2006).
Henry, E.W. & Sidman, R.L. Long lives for homozygous trembler mutant mice despite virtual absence of peripheral nerve myelin. Science 241, 344–346 (1988).
Hesp, Z.C., Goldstein, E.A., Miranda, C.J., Kaspar, B.K. & McTigue, D.M. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J. Neurosci. 35, 1274–1290 (2015).Genetic fate mapping determines that the majority of oligodendrocytes are produced weeks after contusive spinal cord injury. This work highlights that remyelination can continue for months after injury.
Powers, B.E. et al. Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J. Neurosci. 32, 5120–5125 (2012).This is the first study to genetically label new myelin using an inducible membrane-tethered GFP in OPCs after SCI, revealing that the g -ratio is not altered in new myelin following SCI compared to developmental myelin 6 months after injury.
Keirstead, H.S. et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 25, 4694–4705 (2005).Transplanting OPCs increases the number of thinly myelinated axons, suggestive of oligodendrocyte remyelination, and promotes hindlimb motor function. This work was the basis for a clinical trial conducted and terminated early by Geron Corporation and now restarted under Asterias Biotherapeutics.
All, A.H. et al. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One 10, e0116933 (2015).
Cao, Q. et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J. Neurosci. 30, 2989–3001 (2010).
Karimi-Abdolrezaee, S., Eftekharpour, E., Wang, J., Morshead, C.M. & Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 26, 3377–3389 (2006).
Plemel, J.R. et al. Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia 59, 1891–1910 (2011).This paper describes transplantation of PDGF-responsive precursors capable of differentiation into oligodendrocytes and myelinating after SCI, and demonstrates that transplanting cells capable of differentiating into myelinating oligodendrocytes is not sufficient to enhance recovery without an increase in total myelination.
Cao, Q. et al. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J. Neurosci. 25, 6947–6957 (2005).
Hwang, D.H. et al. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 10, 117 (2009).
Hofstetter, C.P. et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 8, 346–353 (2005).This paper presents a cautionary study that demonstrated that NSPC transplantation can result in aberrant axon growth and allodynia. Suppressing astrocyte differentiation of NSPCs prevents allodynia, highlighting the importance of regulating transplanted cell fates.
Hawryluk, G.W.J. et al. An examination of the mechanisms by which neural precursors augment recovery following spinal cord injury: a key role for remyelination. Cell Transplant. 23, 365–380 (2014).
Yasuda, A. et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells 29, 1983–1994 (2011).Transplantation of NSPCs from shiverer mice is associated with substantially less functional improvement compared to wild-type transplanted cells, suggesting compact myelin formation is important for recovery.
Privat, A., Jacque, C., Bourre, J.M., Dupouey, P. & Baumann, N. Absence of the major dense line in myelin of the mutant mouse Shiverer. Neurosci. Lett. 12, 107–112 (1979).
Tang, Y., Stryker, M.P., Alvarez-Buylla, A. & Espinosa, J.S. Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc. Natl. Acad. Sci. USA 111, 18339–18344 (2014).
Salegio, E.A. et al. A unilateral cervical spinal cord contusion injury model in non-human primates (Macaca mulatta). J. Neurotrauma 33, 439–459 (2016).
Nori, S. et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 4, 360–373 (2015).
Zhang, Y. et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J. Neurosci. 33, 12970–12981 (2013).
Meisel, C., Schwab, J.M., Prass, K., Meisel, A. & Dirnagl, U. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 6, 775–786 (2005).
Brommer, B. et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 139, 692–707 (2016).
Kwon, B.K. et al. Demonstrating efficacy in preclinical studies of cellular therapies for spinal cord injury - how much is enough? Exp. Neurol. 248, 30–44 (2013).
Dvorak, M.F. et al. Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: an observational Canadian cohort analysis. J. Neurotrauma 31, 1540–1547 (2014).
Steeves, J.D. et al. Outcome measures for acute/subacute cervical sensorimotor complete (AIS-A) spinal cord injury during a phase 2 clinical trial. Top. Spinal Cord Inj. Rehabil. 18, 1–14 (2012).
Kobayashi, Y. et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One http://dx.doi.org/10.1371/journal.pone.0052787 (2012).
Okada, S. et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834 (2006).
Steeves, J.D. et al. Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord 49, 257–265 (2011).
Nishimura, S. et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol. Brain 6, 3 (2013).
Barakat, D.J. et al. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 14, 225–240 (2005).
Anderson, K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 (2004).
Powers, B.E. et al. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc. Natl. Acad. Sci. USA 110, 4075–4080 (2013).
James, N.D. et al. Conduction failure following spinal cord injury: functional and anatomical changes from acute to chronic stages. J. Neurosci. 31, 18543–18555 (2011).
Blight, A.R. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent. Nerv. Syst. Trauma 2, 299–315 (1985).
Guest, J.D., Hiester, E.D. & Bunge, R.P. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 192, 384–393 (2005).
Bunge, R.P., Puckett, W.R., Becerra, J.L., Marcillo, A. & Quencer, R.M. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–89 (1993).
Karimi-Abdolrezaee, S., Eftekharpour, E., Wang, J., Schut, D. & Fehlings, M.G. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J. Neurosci. 30, 1657–1676 (2010).
McTigue, D.M., Wei, P. & Stokes, B.T. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 21, 3392–3400 (2001).
Tripathi, R. & McTigue, D.M. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia 55, 698–711 (2007).
Lytle, J.M. & Wrathall, J.R. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur. J. Neurosci. 25, 1711–1724 (2007).
Barnabé-Heider, F. et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7, 470–482 (2010).
Whittaker, M.T. et al. GGF2 (Nrg1-beta3) treatment enhances NG2+ cell response and improves functional recovery after spinal cord injury. Glia 60, 281–294 (2012).
Schonberg, D.L. et al. Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo. J. Neurosci. 32, 5374–5384 (2012).
Goritz, C. et al. A pericyte origin of spinal cord scar tissue. Science 333, 238–242 (2011).
Meletis, K. et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 6, e182 (2008).
Ren, Y. et al. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dpeendent on direct ependymal injury. Sci. Rep. 7, 41122 (2017).
Soderblom, C. et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 33, 13882–13887 (2013).
Acknowledgements
P.A. received a Canadian Institute for Health Research (CIHR) Frederick Banting and Charles Best Canadian Graduate Scholarship–Doctoral Award and the CIHR Transplantation Training Program Award. G.J.D. was supported by a Multiple Sclerosis Society of Canada Doctoral Scholarship and the CIHR Transplantation Training Program Award. B.J.H. holds a CIHR Frederick Banting and Charles Best Canadian Graduate Scholarship–Doctoral Award, a Zoology Graduate Fellowship and a CIHR Transplantation Training Program Award. J.R.P. is supported by the Donna Joan Oxford Postdoctoral Fellowship Award from the Multiple Sclerosis Society of Canada and by postdoctoral fellowship awards from CIHR, Alberta Innovates Health Solutions and T. Chen Fong. W.T. receives support from the John and Penny Ryan British Columbia Leadership Chair in Spinal Cord Research and from CIHR FRN 130475.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Assinck, P., Duncan, G., Hilton, B. et al. Cell transplantation therapy for spinal cord injury. Nat Neurosci 20, 637–647 (2017). https://doi.org/10.1038/nn.4541
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4541
This article is cited by
-
Efficacy of the immediate adipose-derived stromal vascular fraction autograft on functional sensorimotor recovery after spinal cord contusion in rats
Stem Cell Research & Therapy (2024)
-
Interleukin-4 from curcumin-activated OECs emerges as a central modulator for increasing M2 polarization of microglia/macrophage in OEC anti-inflammatory activity for functional repair of spinal cord injury
Cell Communication and Signaling (2024)
-
Biomaterial-based regenerative therapeutic strategies for spinal cord injury
NPG Asia Materials (2024)
-
M2 Microglia-derived Exosomes Promote Spinal Cord Injury Recovery in Mice by Alleviating A1 Astrocyte Activation
Molecular Neurobiology (2024)
-
BMSC-Derived Exosomes Carrying miR-26a-5p Ameliorate Spinal Cord Injury via Negatively Regulating EZH2 and Activating the BDNF-TrkB-CREB Signaling
Molecular Neurobiology (2024)