Abstract
Push–pull is a canonical computation of excitatory cortical circuits. By contrast, we identify a pull–push inhibitory circuit in frontal cortex that originates in vasoactive intestinal polypeptide (VIP)-expressing interneurons. During arousal, VIP cells rapidly and directly inhibit pyramidal neurons; VIP cells also indirectly excite these pyramidal neurons via parallel disinhibition. Thus, arousal exerts a feedback pull–push influence on excitatory neurons—an inversion of the canonical push–pull of feedforward input.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chen, T.W. et al. Nature 499, 295–300 (2013).
McGinley, M.J., David, S.V. & McCormick, D.A. Neuron 87, 179–192 (2015).
Ebitz, R.B. & Platt, M.L. Neuron 85, 628–640 (2015).
Zekveld, A.A., Heslenfeld, D.J., Johnsrude, I.S., Versfeld, N.J. & Kramer, S.E. Neuroimage 101, 76–86 (2014).
Vinck, M., Batista-Brito, R., Knoblich, U. & Cardin, J.A. Neuron 86, 740–754 (2015).
Niell, C.M. & Stryker, M.P. Neuron 65, 472–479 (2010).
Polack, P.O., Friedman, J. & Golshani, P. Nat. Neurosci. 16, 1331–1339 (2013).
Mineault, P.J., Tring, E., Trachtenberg, J.T. & Ringach, D.L. J. Neurosci. 36, 6382–6392 (2016).
Letzkus, J.J., Wolff, S.B. & Lüthi, A. Neuron 88, 264–276 (2015).
Pfeffer, C.K., Xue, M., He, M., Huang, Z.J. & Scanziani, M. Nat. Neurosci. 16, 1068–1076 (2013).
Lee, S., Kruglikov, I., Huang, Z.J., Fishell, G. & Rudy, B. Nat. Neurosci. 16, 1662–1670 (2013).
Pi, H.J. et al. Nature 503, 521–524 (2013).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Nat. Neurosci. 10, 663–668 (2007).
Karnani, M.M. et al. J. Neurosci. 36, 3471–3480 (2016).
Fu, Y. et al. Cell 156, 1139–1152 (2014).
Anderson, J.S., Carandini, M. & Ferster, D. J. Neurophysiol. 84, 909–926 (2000).
Douglas, R.J. & Martin, K.A. J. Physiol. (Lond.) 440, 735–769 (1991).
Huber, D. et al. Nature 484, 473–478 (2012).
Greenberg, D.S. & Kerr, J.N. J. Neurosci. Methods 176, 1–15 (2009).
Smith, S.L. & Häusser, M. Nat. Neurosci. 13, 1144–1149 (2010).
Ozden, I., Lee, H.M., Sullivan, M.R. & Wang, S.S. J. Neurophysiol. 100, 495–503 (2008).
Acknowledgements
This work was funded by R01 EY023871 (JTT), R01 EY018322 (DLR) and R01 EB022915 (DLR). PGJC was supported by postdoctoral Fellowship EX2009-0750 from the Spanish Ministry of Education, Culture and Sport and by postdoctoral contract from Junta de Andalucía (P12-CTS-2232).
Author information
Authors and Affiliations
Contributions
P.G.-J.-C. collected and analyzed the data in Figures 1 and 2 and Supplementary Figures 1, 2, 3. D.L.R. analyzed the covariance data in Figure 2. E.T., D.L.R. and J.T.T. contributed to the in vivo photostimulation experiments in Figure 3. T.I. and X.X. contributed to the slice ChR2 mapping data in Figure 3.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
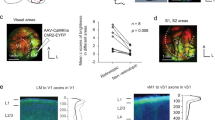
Supplementary Figure 1 Overview of experimental setup.
(a) Schematic representation of the approach to viral infection and in vivo imaging. (b)Top: diagram of the genetic cross used to generate mice in which all inhibitory neurons expressed tdTomato. Middle left: cropped image showing GCaMP6 and tdTomato expression in FrA. Bottom left: z-scored time series GCaMP6s responses for 50 excitatory neurons imaged over 10 minutes. Middle right: example traces from 3 excitatory neurons and 2 inhibitory neurons. Traces are z-scored. Excitatory neurons show fast, large amplitude responses, whereas inhibitory neurons show slower, smaller amplitude responses. Bottom right: z-scored traces of ball motion (gray) and pupil diameter (black) imaged over 10 minutes. Note that as locomotion increases, so does pupil diameter.
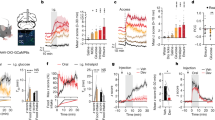
Supplementary Figure 2 Responses of excitatory neurons expressing GCaMP6-fast.
(a) Ordinate: plot of the correlation coefficients between excitatory response and locomotion. Abscissa: plot of the correlation coefficients between those same neurons and pupil diameter. All neurons recorded are plotted. Note that the activities of many excitatory neurons are suppressed by locomotion and increased pupil diameter. n=3248 neurons, 11 mice, 22 fields of view. (b) Histograms plotting excitatory correlation coefficients to running (top) and pupil diameter (bottom). A Gaussian distribution was fitted to each histogram (black dashed line). Gray lines in both show the cumulative distributions. Note that the coefficients are normally distributed, with roughly half of responses showing negative coefficients. (c) Example traces from 5 excitatory neurons whose activities are negatively (red, left), positively (green, middle), or un-correlated (blue, right) with locomotion/pupil diameter. Cells were taken from the pool of neurons shown in the colored circles in panel a.
Supplementary Figure 3 GCAMP6-slow responses of VIP and SOM interneurons.
Top 4 panels: SOM cell responses.
(a) z-scored time series GCaMP6s responses in 41 SOM cells imaged over 7 minutes. The black trace at the top plots represents the z-scored running speed. (b) Plot of correlation coefficients between GCaMP6s response and running speed (ordinate) and pupil diameter (abscissa). Responses of 2 individual SOM cells are shown in the green and red traces. n=417 neurons, 7 mice, 12 fields of view. (c) Histograms of correlation coefficients between neural response and pupil diameter (left) and running speed (right). Both plots are fitted with a Gaussian (black dashed lines), and the cumulative distributions are plotted (gray lines). Note that SOM cells are strongly left-shifted in their responses with the clear majority negatively correlated with arousal.
Bottom 4 panels: VIP cell responses.
(d) z-scored time series GCaMP6s responses in 44 VIP cells imaged over 6 minutes. Again, the black trace at the top plots is the z-scored running speed. (e-f) Same plots as in panels b-c, but for VIP cells. Note the opposite response of VIP cells to arousal. n=875 neurons, 7 mice, 16 fields of view. (g) The Gaussian distributions plotted in panels c and f are over-plotted to show more clearly the opposite responsiveness of the SOM (red) and VIP (blue) cells during heightened arousal.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 to 3 (PDF 982 kb)
Rights and permissions
About this article
Cite this article
Garcia-Junco-Clemente, P., Ikrar, T., Tring, E. et al. An inhibitory pull–push circuit in frontal cortex. Nat Neurosci 20, 389–392 (2017). https://doi.org/10.1038/nn.4483
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4483
This article is cited by
-
GABAergic CA1 neurons are more stable following context changes than glutamatergic cells
Scientific Reports (2022)
-
Cortical VIP+ Interneurons in the Upper and Deeper Layers Are Transcriptionally Distinct
Journal of Molecular Neuroscience (2022)
-
Longitudinal functional imaging of VIP interneurons reveals sup-population specific effects of stroke that are rescued with chemogenetic therapy
Nature Communications (2021)
-
Prefrontal somatostatin interneurons encode fear memory
Nature Neuroscience (2020)
-
Polysynaptic inhibition between striatal cholinergic interneurons shapes their network activity patterns in a dopamine-dependent manner
Nature Communications (2020)