Abstract
Agouti-related peptide (AgRP) neurons of the arcuate nucleus of the hypothalamus (ARC) promote homeostatic feeding at times of caloric insufficiency, yet they are rapidly suppressed by food-related sensory cues before ingestion. Here we identify a highly selective inhibitory afferent to AgRP neurons that serves as a neural determinant of this rapid modulation. Specifically, GABAergic projections arising from the ventral compartment of the dorsomedial nucleus of the hypothalamus (vDMH) contribute to the preconsummatory modulation of ARCAgRP neurons. In a manner reciprocal to ARCAgRP neurons, ARC-projecting leptin receptor-expressing GABAergic vDMH neurons exhibit rapid activation upon availability of food that additionally reflects the relative value of the food. Thus, leptin receptor-expressing GABAergic vDMH neurons form part of the sensory network that relays real-time information about the nature and availability of food to dynamically modulate ARCAgRP neuron activity and feeding behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
26 September 2016
In the version of this article initially published online, ref. 7 was cited in the sentence "Furthermore, and as observed of ARCAgRP neurons7, vDMHLepR neuron fluorometric responses to chocolate were significantly greater than to similarly sized chow pellets"; this should have been ref. 8. The error has been corrected for the print, PDF and HTML versions of this article.
References
Sternson, S.M., Nicholas Betley, J. & Cao, Z.F. Neural circuits and motivational processes for hunger. Curr. Opin. Neurobiol. 23, 353–360 (2013).
Aponte, Y., Atasoy, D. & Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Krashes, M.J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Luquet, S., Perez, F.A., Hnasko, T.S. & Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Mandelblat-Cerf, Y. et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife http://dx.doi.org/10.7554/eLife.07122 (2015).
Dietrich, M.O., Zimmer, M.R., Bober, J. & Horvath, T.L. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160, 1222–1232 (2015).
Betley, J.N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Chen, Y., Lin, Y.C., Kuo, T.W. & Knight, Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Chen, Y. & Knight, Z.A. Making sense of the sensory regulation of hunger neurons. BioEssays 38, 316–324 (2016).
Pinto, S. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304, 110–115 (2004).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Wickersham, I.R., Finke, S., Conzelmann, K.K. & Callaway, E.M. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4, 47–49 (2007).
Krashes, M.J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014).
Wang, D. et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat. 9, 40 (2015).
van den Pol, A.N. et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J. Neurosci. 29, 4622–4639 (2009).
Hahn, T.M., Breininger, J.F., Baskin, D.G. & Schwartz, M.W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 (1998).
Betley, J.N., Cao, Z.F., Ritola, K.D. & Sternson, S.M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Rodgers, R.J., Holch, P. & Tallett, A.J. Behavioural satiety sequence (BSS): separating wheat from chaff in the behavioural pharmacology of appetite. Pharmacol. Biochem. Behav. 97, 3–14 (2010).
Chen, T.W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Enriori, P.J., Sinnayah, P., Simonds, S.E., Garcia Rudaz, C. & Cowley, M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 31, 12189–12197 (2011).
Rezai-Zadeh, K. et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 3, 681–693 (2014).
Simonds, S.E. et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416 (2014).
Allison, M.B. et al. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol. Metab. 4, 299–309 (2015).
Henry, F.E., Sugino, K., Tozer, A., Branco, T. & Sternson, S.M. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife http://dx.doi.org/10.7554/eLife.09800 (2015).
Knight, Z.A. et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151, 1126–1137 (2012).
Cao, W.H. & Morrison, S.F. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology 51, 426–437 (2006).
Fontes, M.A., Tagawa, T., Polson, J.W., Cavanagh, S.J. & Dampney, R.A. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am. J. Physiol. Heart Circ. Physiol. 280, H2891–H2901 (2001).
Shek, E.W., Brands, M.W. & Hall, J.E. Chronic leptin infusion increases arterial pressure. Hypertension 31, 409–414 (1998).
Leshan, R.L., Björnholm, M., Münzberg, H. & Myers, M.G. Jr. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 14 (Suppl. 5), 208S–212S (2006).
Parton, L.E. et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449, 228–232 (2007).
Garfield, A.S. et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 18, 863–871 (2015).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Atasoy, D., Aponte, Y., Su, H.H. & Sternson, S.M. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Franklin, B.J.P.G. The Mouse Brain in Stereotaxic Coordinates (Academic Press, 2008).
Acknowledgements
We thank B. Sabatini and K.W. Huang for assistance with in vivo fiber photometry. We thank Drs. V. Jayaraman, R.A. Kerr, D.S. Kim, L.L. Looger and K. Svoboda and the GENIE Project at Janelia Farm Research Campus, Howard Hughes Medical Institute, for distribution of GCaMP6. This work was supported by the University of Edinburgh Chancellor's Fellowship (A.S.G.); National Institutes of Health grants R01 DK096010 (B.B.L.), R01 DK089044 (B.B.L.), R01 DK071051 (B.B.L.), R01 DK075632 (B.B.L.), R37 DK053477 (B.B.L.), BNORC Transgenic Core P30 DK046200 (B.B.L.), R01 DK056731 (M.J.G.), BADERC Transgenic Core P30 DK057521 (B.B.L.), F32 DK089710 (M.J.K.), DP2 DK105570-01 (M.L.A.) and R01 DK109930 (M.L.A.); the McKnight Foundation (M.L.A.); the Klarman Family Foundation (M.L.A.); the Richard and Susan Smith Family Foundation (M.L.A.); the Pew Scholars Program in Biomedical Sciences (M.L.A.); ADA Mentor-Based Fellowship (B.P.S. and B.B.L.); Davis Family Foundation postdoctoral fellowship award (C.R.B.); and AHA postdoctoral fellowship 14POST20100011 (J.N.C.). This research was supported, in part, by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK075087, DK075088). The GH Viral vector core facility was funded by NIH/NINDS grant P30NS045776 (B.A.T.) for the preparation of the rabies virus.
Author information
Authors and Affiliations
Contributions
A.S.G., B.P.S., M.J.K. and B.B.L. conceived the studies. A.S.G., B.P.S., C.R.B., M.M.L. and M.J.K. conducted the studies with assistance from C.L., J.S.S., J.C.M., D.K. and B.A.T. Photometry experiments and analysis were conducted by C.R.B., M.M.L. and M.L.A. Single cell qPCR analysis was conducted by J.N.C. A.S.G. and B.B.L. wrote the manuscript with assistance from B.P.S., C.R.B., M.M.L., M.J.K., M.G.M. and T.E.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
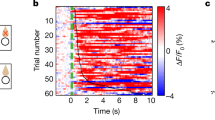
Supplementary Figure 1 Analysis of GABAergic afferents to ARCAgRP and ARCPOMC neurons
a-c, ARC-projecting vGAT afferents arising from DMH, ARC and LH. d-l, CRACM analysis of monosynaptic afferents from vGAT- or LepR-expressing neurons in the ARC and LH. d-e, 100% of recorded ARCAgRP neurons receive GABAergic input from local ARCvGAT neurons (d) but not distal LHvGAT neurons (e). f-g, 100% of recorded ARCPOMC neurons receive GABAergic input from local ARCvGAT neurons (f) but not distal LHvGAT neurons (g). h, DMHLepR→ARC neurons do not engage non-AgRP neurons. i-m, 100% recorded ARCAgRP (i) and ARCPOMC neurons (j-k) receive GABAergic input from local ARCLepR neurons but not LHLepR neurons (l-m). Abbreviations, 3v, third ventricle; PTX, picrotoxin. Scale bar in a, 100 μm and applies to all images.
Supplementary Figure 2 GABAergic DMHLepR neurons are localized to the ventral DMH
a, GABAergic (vGAT-expressing; green) leptin responsive DMH neurons, as demarked by pSTAT3-immunoreactivty (red), are predominantly localized to the ventral DMH. b, glutamatergic (vGLUT2-expressing; green) pSTAT3-immunoreacitve (red) neurons are predominantly localized to the dorsal hypothalamic area (DHA) and dorsal DMH. c, leptin responsive EnvA-EGFP-labeled ARCAgRP afferents within the DMH are localized to the ventral DMH. White arrows denote GFP and pSTAT3 colocalization. Abbreviations, dDMH, dorsal DMH; DHA, dorsal hypothalamic area; DMC, DMH central part; vDMH, ventral DMH. Scale bar in a, b and d, 100 μm; in a’, a’’, b’ and b’’, 50 μm; in d’, 30 μm. Each experiment was reproduced in 2-3 mice.
Supplementary Figure 3 vDMHLepR→ARC neurons do not collateralize to other efferent sites
a, histological sections throughout the rostral-caudal extent of the mouse brain demonstrating the efferent projections of DMHLepR neurons (labelled with ChR2-mCherry) to the lateral septum, ventral part (LSV); bed nucleus of the stria terminalis, lateroventral part; paraventricular nucleus of the hypothalamus (PVH); arcuate nucleus (ARC); ventrolateral periaqueductal grey (vlPAG); pre-locus coeruleus (pLC) and raphe pallidus (RPa). b, Rabies collateral mapping suggests that DMHLepR→ARC neurons do not collateralise to any efferent sites. c, in the absence of the TVA-mCherry helper virus no EnvA-GFP infectivity is observed, demonstrating the necessity of TVA for cellular entry of the EnvA virus. Abbreviations, 3v, third ventricle; aca, anterior commissure, anterior part; aq, aqueduct; cc, corpus collosum; CPu, caudate putamen; dPAG, dorsal periaqueductal grey; DRD, dorsal raphe nucleus, dorsal part; py, pyramidal tract; scp, superior cerebellar peduncle; VMH, ventromedial nucleus of the hypothalamus. Scale bar 100 μm and applies to all images. Each experiment was reproduced in 2-3 mice.
Supplementary Figure 4 Optogenetic stimulation of vDMHLepR→ARC terminals does not affect general locomotor behavior
a, representative histological image of bilateral optical fiber placement for photostimulation of DMHLepR→ARC terminals. b, photostimulation of ChR2-mCherry expressing DMHLepR →ARC terminals 10 seconds or 10 minutes prior to food presentation reduced food consumption to the same extent, compared to laser off condition (n=6-8; one way ANOVA, F(2,15)=72.89, p<0.0001; post-doc: OFF v ON (10 s), ****p<0.0001; OFF v ON (10 m), ****p<0.0001; ON (10 s) v ON (10 m), p=0.94). c, photostimulation of DMHLepR →ARC terminals in the absence of ChR2-mCherry (transduced with cre-dependent GFP) does not influence dark cycle food intake (n=4; repeated measures ANOVA, main effect of treatment and interaction, not significant, main effect of time (F(3,12)=84.95, p<0.0001). d, photostimulation of DMHLepR→ARC terminals in a dark cycle paradigm was sufficient to inhibit food intake when applied before or after food consumption had begun (n=6, repeated measures ANOVA, F(5,10)=15.52, p=0.011; post-hoc compared to ‘OFF’: ON (Before), *p=0.02; ON (After), *p=0.03). e-f, photostimulation of DMHLepR→ARC terminals does not significantly affect homecage locomotor activity in during a 3 hour dark cycle paradigm (e; n=4, paired t-test, t(3)=0.88, p=0.44) or 1 hour light cycle paradigm (f; n=6, paired t-test, t(5)=0.59, p=0.60) of food. g-i, photostimulation of DMHLepR→ARC terminals does not significantly affect the time spent at the edge (g; n=6, paired t-test, t(5)=0.03, p=0.97), the center (g; paired t-test, t(5)=0.19, p=0.85) or the total distance travelled (h; paired t-test, t(5)=0.34, p=0.74) in a novel open-field arena. j, photostimulation of DMHLepR→ARC terminals significantly increased grooming to a level comparable to that seen following food consumption (n=6, repeated measures ANOVA, F(5,10)=23.51, p=0.0015; post-hoc: Fasted v Refed, ***p=0.0009; Fasted v Fasted+laser, **p=0.004; Refed v Fasted+laser, p=0.31). All data presented as mean±SEM. Abbreviations, 3v, third ventricle.
Supplementary Figure 5 vDMHLepR→ARC neurons are not necessary for the maintenance of satiety
a-b, DMHLepR neurons were transduced with cre-dependent inhibitory DREADD (hM4Di-mCherry). c, membrane potential and firing rate of LepR-ires-Cre::hM4Di-mCherryDMH neurons decreased upon application of 5 µM CNO during electrophysiological current clamp recordings from acute slices. d, chemogenetic inhibition of DMHLepR neurons does not effect light-cycle food intake (n=7; repeated measures ANOVA, main effect of treatment and interaction, not significant, main effect of time (F(3,24)=43.61, p<0.0001). All data presented as mean±SEM. Scale bar in b, 100 μm.
Supplementary Figure 6 Calcium responses in vDMHLepR neurons are specific to food presentation
a-b, neither food nor object presentation elicited changes in vivo fluorescence in mice with Cre-dependent expression of AAV-DIO-GFP in DMHLepR neurons (a, mean effects from all mice across time, n=3; b, mean response from 0-10s post food presentation, paired t-test, t(2)=0.41, p=0.71). c-d, mice with validated ‘misses’ in which cre-dependent GCaMP6s was expressed predominantly in the ventromedial nucleus of the hypothalamus LepR-expressing neurons exhibited spontaneous calcium transients but no response to food presentation, compared to a non-food item (c, mean response across all mice across time, n=3; d, mean response from 0-10s post food presentation, paired t-test, t(2)=1.43, p=0.29). All data presented as mean±SEM.
Supplementary Figure 7 vDMHLepR→ARC axons respond to food quality
a-b, DMHLepR→ARC axons were rapidly activated upon presentation of a large chow pellet, compared to a non-food object, in a energy-state dependent manner (a, mean effects from all mice across time, n=6; b, mean response from 0-10s post food presentation, repeated measures ANOVA, F(5,15)=12.71, p=0.0033; post-hoc: Fasted – Obj v Chow, **p=0.012; Fed – Obj v Chow, p=0.335; Fasted chow v fed chow, *p=0.02). c-d, presentation of chocolate activated DMHLepR→ARC axons, compared to a non-food object, in both the food restricted and ad libitum fed state (c, mean effects from all mice across time, n=6; d, mean response from 0-10s post food presentation, repeated measures ANOVA, F(4,12)=27.23, p=0.0007; post-hoc: Fasted – Obj v Choc, **p=0.008; Fed – Obj v Choc, *p=0.04; Fasted choc v Fed choc, *p=0.04). All data presented as mean±SEM.
Supplementary Figure 8 vDMHpDYN neurons are a subset of GABAergic vDMHLepR→ARC neurons
a-b, Single cell picking (a) and quantitative PCR (b) of 25 genetically labeled DMHLepR neurons revealed that a subset of GABAergic DMHLepR neurons express pDYN (14/25). c-e, DMHpDYN neurons, demarked by a pDYN-ires-Cre::L10-GFP mouse line, in the ventral DMH (green) are leptin responsive, as evinced by pSTAT3-immunoreactivity (red); (white arrows denote GFP and pSTAT3 colocalization). f-h DMHpDYN neurons project to the arcuate nucleus of the hypothalamus and innervate ARCAgRP neurons. i, DMHpDYN neurons do not make monosynaptic connections with ARCnon-AgRP neurons (0/12 connected). j, the amplitude of DMHpDYN→ARCAgRP light-evoked IPSCs is significantly smaller than DMHLepR→ARCAgRP light-evoked IPSCs (n=20-29, two-tailed t-test, t(47)=4.55, p<0.0001). l, photosimulation of DMHpDYN→ARC terminals was sufficient to inhibit ARCAgRP action potential firing in some (representative cell 1, 2/4 neurons), but not all, ARCAgRP neurons (representative cell 2, 2/4 neurons). All data presented as mean±SEM, ****p<0.0001. Abbreviations, ARC, arcuate nucleus of the hypothalamus; dDMH, dorsal DMH; DHA, dorsal hypothalamic area; DMC, DMH central part; vDMH, ventral DMH; PTX, picrotoxin. Scale bar in c and f, 200 μm; in d, e, g, 100 μm and in h 20 μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1931 kb)
DMHLepR→ARCAgRP are sufficient to inhibit post-fast refeeding
Afferent inhibition of ARCAgRP neurons through in vivo optogenetic stimulation of DMHLepR→ARC neurons is sufficient to inhibit food seeking and consumption in a post-fast refeed paradigm. (MOV 16602 kb)
Rights and permissions
About this article
Cite this article
Garfield, A., Shah, B., Burgess, C. et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci 19, 1628–1635 (2016). https://doi.org/10.1038/nn.4392
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4392
This article is cited by
-
Daily feeding entrains hunger-sensing neurons
Nature Neuroscience (2024)
-
AgRP neurons encode circadian feeding time
Nature Neuroscience (2024)
-
A sex-specific thermogenic neurocircuit induced by predator smell recruiting cholecystokinin neurons in the dorsomedial hypothalamus
Nature Communications (2023)
-
High sucrose consumption decouples intrinsic and synaptic excitability of AgRP neurons without altering body weight
International Journal of Obesity (2023)
-
Advanced neurobiological tools to interrogate metabolism
Nature Reviews Endocrinology (2023)