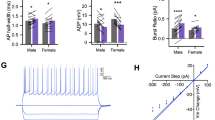
Abstract
Social encounters are associated with varying degrees of emotional arousal and stress. The mechanisms underlying adequate socioemotional balance are unknown. The medial amygdala (MeA) is a brain region associated with social behavior in mice. Corticotropin-releasing factor receptor type-2 (CRF-R2) and its specific ligand urocortin-3 (Ucn3), known components of the behavioral stress response system, are highly expressed in the MeA. Here we show that mice deficient in CRF-R2 or Ucn3 exhibit abnormally low preference for novel conspecifics. MeA-specific knockdown of Crfr2 (Crhr2) in adulthood recapitulated this phenotype. In contrast, pharmacological activation of MeA CRF-R2 or optogenetic activation of MeA Ucn3 neurons increased preference for novel mice. Furthermore, chemogenetic inhibition of MeA Ucn3 neurons elicited pro-social behavior in freely behaving groups of mice without affecting their hierarchal structure. These findings collectively suggest that the MeA Ucn3–CRF-R2 system modulates the ability of mice to cope with social challenges.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Tost, H., Champagne, F.A. & Meyer-Lindenberg, A. Environmental influence in the brain, human welfare and mental health. Nat. Neurosci. 18, 1421–1431 (2015).
Iarocci, G., Yager, J. & Elfers, T. What gene-environment interactions can tell us about social competence in typical and atypical populations. Brain Cogn. 65, 112–127 (2007).
Heimberg, R.G. et al. Social anxiety disorder in DSM-5. Depress. Anxiety 31, 472–479 (2014).
Mehling, M.H. & Tassé, M.J. Severity of autism spectrum disorders: current conceptualization, and transition to DSM-5. J. Autism Dev. Disord. 46, 2000–2016 (2016).
Eisenberger, N.I. & Cole, S.W. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci. 15, 669–674 (2012).
Sztainberg, Y. & Chen, A. Neuropeptide regulation of stress-induced behavior: insights from the crf/urocortin family. in Handbook of Neuroendocrinology (eds Fink, G., Pfaff, D.W. & Levine, J.) Ch. 15 (Academic Press, 2011).
Lewis, K. et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA 98, 7570–7575 (2001).
Deussing, J.M. et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J. Neurosci. 30, 9103–9116 (2010).
Van Pett, K. et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 428, 191–212 (2000).
Goodson, J.L. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22 (2005).
Brennan, P.A. & Keverne, E.B. Something in the air? New insights into mammalian pheromones. Curr. Biol. 14, R81–R89 (2004).
Samuelsen, C.L. & Meredith, M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 1263, 33–42 (2009).
Toth, I. & Neumann, I.D. Animal models of social avoidance and social fear. Cell Tissue Res. 354, 107–118 (2013).
Silverman, J.L., Yang, M., Lord, C. & Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11, 490–502 (2010).
Gray, S.J., Jensen, S.P. & Hurst, J.L. Structural complexity of territories: preference, use of space and defence in commensal house mice, Mus domesticus. Anim. Behav. 60, 765–772 (2000).
Kalueff, A.V., Wheaton, M. & Murphy, D.L. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 179, 1–18 (2007).
Shemesh, Y. et al. High-order social interactions in groups of mice. Elife 2, e00759 (2013).
de Chaumont, F. et al. Computerized video analysis of social interactions in mice. Nat. Methods 9, 410–417 (2012).
Hong, W. et al. Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc. Natl. Acad. Sci. USA 112, E5351–E5360 (2015).
Moy, S.S. et al. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 8, 129–142 (2009).
Watabe-Uchida, M., Zhu, L., Ogawa, S.K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012).
Allen Institute for Brain Science. Allen Mouse Brain Atlas. http://mouse.brain-map.org (2015).
Lein, E.S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007).
Wesson, D.W. & Wilson, D.A. Sniffing out the contributions of the olfactory tubercle to the sense of smell: hedonics, sensory integration, and more? Neurosci. Biobehav. Rev. 35, 655–668 (2011).
Lebow, M.A. & Chen, A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016).
Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 76, 142–159 (2012).
Lo, L. & Anderson, D.J.A. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950 (2011).
Pardo-Bellver, C., Cádiz-Moretti, B., Novejarque, A., Martínez-García, F. & Lanuza, E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front. Neuroanat. 6, 33 (2012).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Kirouac, G.J. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev. 56, 315–329 (2015).
Lebow, M. et al. Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J. Neurosci. 32, 6906–6916 (2012).
Keshavarzi, S., Sullivan, R.K., Ianno, D.J. & Sah, P. Functional properties and projections of neurons in the medial amygdala. J. Neurosci. 34, 8699–8715 (2014).
Elliott, E., Ezra-Nevo, G., Regev, L., Neufeld-Cohen, A. & Chen, A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat. Neurosci. 13, 1351–1353 (2010).
Peça, J. et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472, 437–442 (2011).
Urban, D.J. & Roth, B.L. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu. Rev. Pharmacol. Toxicol. 55, 399–417 (2015).
Bronson, F.H. The reproductive ecology of the house mouse. Q. Rev. Biol. 54, 265–299 (1979).
Selten, J.-P., van der Ven, E., Rutten, B.P. & Cantor-Graae, E. The social defeat hypothesis of schizophrenia: an update. Schizophr. Bull. 39, 1180–1186 (2013).
van Os, J., Kenis, G. & Rutten, B.P. The environment and schizophrenia. Nature 468, 203–212 (2010).
Anderson, D.J. & Perona, P. Toward a science of computational ethology. Neuron 84, 18–31 (2014).
De Dreu, C.K., Greer, L.L., Van Kleef, G.A., Shalvi, S. & Handgraaf, M.J. Oxytocin promotes human ethnocentrism. Proc. Natl. Acad. Sci. USA 108, 1262–1266 (2011).
Miskovic, V. & Schmidt, L.A. Social fearfulness in the human brain. Neurosci. Biobehav. Rev. 36, 459–478 (2012).
Rabiner, L.R. A tutorial on hidden Markov models and selected applications in speech recognition. Proc. IEEE 77, 257–286 (1989).
Duda, R.O., Hart, P.E. & Stork, D.G. Pattern Classification and Scene Analysis 2nd ed. (Wiley Interscience, 1995).
Breiman, L., Friedman, J., Stone, C.J. & Olshen, R.A. Classification and Regression Trees (CRC Press, 1984).
Clopper, C.J. & Pearson, E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26, 404–413 (1934).
Cormen, T.H. Introduction to Algorithms (MIT Press, 2009).
de Vries, H., Stevens, J.M. & Vervaecke, H. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592 (2006).
Howell, D. Statistical Methods for Psychology (Cengage Learning, 2012).
Acknowledgements
We thank S. Ovadia for his devoted assistance with animal care. We thank J. Keverne for professional English editing, formatting and scientific input. This work is supported by an FP7 grant from the European Research Council (260463; A.C.); research grants from the Israel Science Foundation (1565/15) (A.C.); research support from Roberto and Renata Ruhman (A.C.); research support from Bruno and Simone Licht; I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant no. 1916/12 to A.C.); the Nella and Leon Benoziyo Center for Neurological Diseases (A.C.); the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics (A.C.); the Perlman Family Foundation, founded by Louis L. and Anita M. Perlman (A.C.); the Adelis Foundation (A.C.); the Irving I. Moskowitz Foundation (A.C.); grants from the Israel Science Foundation (1351/12) and the European Commission (ERC StG #337637 and Marie Curie CIG #321919) (O.Y.) and a Human Frontier Program career development award (O.Y.); a Human Frontier Science Program grant (E.S.); European Research Council grant # 311238 (E.S.); an Israel Science Foundation grant #1629/12 (E.S.); research support from Martin Kushner Schnur (E.S.); and Mr. and Mrs. Lawrence Feis (E.S.).
Author information
Authors and Affiliations
Contributions
Y. Shemesh and O.F. designed and performed most of the experiments. M.M., M.E., J.D., and J.M.D. designed and performed electrophysiological studies. S.A., S.M., T.S., E.E., L.T., G.E., E.S.A., Y.J.B.-E., S.G., Y.K., and S.H. assisted in experiments. Y. Shemesh, Y. Sztainberg, and O.F. wrote the code for analyzing behavior and validated it. E.S., O.Y., and A.C. conceived, designed, and supervised the project. Y. Shemesh, O.F., and A.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Crfr2 KO and Ucn3 KO mice were similar to WT mice in their nonsocial exploratory behavior
(a) Schematic illustration of the social approach test. (b) An illustration of the novel object preference test. (c) Exploratory behavior (number of visits to each chamber in the social approach test) of Crfr2 KO mice (n=7) and WT littermate controls (n=8) did not differ (two-tailed t-test, p=0.1907, t=1.380, df=13). Exploratory behavior (number of visits to each chamber) of Ucn3 KO mice (n=6) and WT littermate controls (n=7) did not differ (two-tailed t-test, p=0.7824, t=0.283, df=11). (d) Average speed of Crfr2 KO mice (n=7) and WT littermate controls (n=8) in the social maze did not differ (two-tailed t-test, p=0.3394, t=0.9918, df=13). Average speed of Ucn3 KO mice (n=6) and WT littermate controls (n=7) in the social maze did not differ (two-tailed t-test, p=0.1611, t=1.502, df=11). Bars represent mean ± s.e.m.
Supplementary Figure 2 Validation of the Crfr2::Cre line
Mice expressing Cre-recombinase under the control of the Crfr2 promoter were crossed with a line carrying a reporter allele encoding the tdTomato red fluorescent protein upon Cre-mediated recombination (Rosa26-CAG-lsl-tdtomato, Ai9). Red cells represent cells that expressed CRF-R2 at any point of time from early development (n=4 mice).
Supplementary Figure 3 Validation of the Ucn3::Cre line
Mice expressing Cre-recombinase under the control of the Ucn3 promoter were crossed with a line carrying a reporter allele encoding the tdTomato red fluorescent protein upon Cre-mediated recombination (Rosa26-CAG-lsl-tdtomato, Ai9). To obtain the best immunostaining we used colchicine-injected mice. Images depict the co-localization of anti-Ucn3 antibody (green) with Ai9 fluorescence (red) in regions that typically express Ucn3 (PeF, BNST, MeA). As expected, the anti-Ucn3 is highly specific to the Ai9 cells, 90±5 % of the green cells are red (averaged over all 3 areas in 3 different mice). Since not all Ai9 expressing cells produced Ucn3 at the time of sacrifice, many of the red cells are not green.
Supplementary Figure 4 The MeA Ucn3–CRF-R2 circuit is embedded within the social brain network
(a) Injection site (MeA) of the rabies, herpes and conditional synaptophysin viruses. (b) Monosynaptic inputs (green cells) to MeA Ucn3 neurons. Ucn3::Cre mice were injected with conditional helpers (AAV5-FLEX-TVA-mCherry, AAV8-FLEX-RG, and a conditional rabies virus SADΔG-GFP (EnvA)). (c) Synaptic outputs from MeA Ucn3 neurons. Conditional Cre dependent anterograde transneuronal tracer based on the H129 strain of herpes simplex virus (H129 TK-TT) was delivered to MeA Ucn3 cells. Red cells indicate the tdTomato marker expressed in cells anterograde to MeA Ucn3::Cre expressing cells. (d) Efferent projections from MeA CRF-R2 cells. An AAV9-CMV-Flex-synaptophysin-mCherry viral vector was injected bilaterally into the MeA of Crfr2::Cre mice. Red dots represent synapses that originated from MeA CRF-R2 neurons.
Supplementary Figure 5 Crfr2 KD mice were similar to control mice in their nonsocial exploratory behavior
(a) Exploratory behavior (number of visits to each chamber in the social approach test) of control mice (n=10), MeA Crfr2 KD mice (n=10) and LS Crfr2 KD mice (n=6) did not differ (two-tailed t-test, p=0.6891, F(2,23)=0.3785). (b) Average speed of control mice (n=10), MeA Crfr2 KD mice (n=10) and LS Crfr2 KD mice (n=6) did not differ (two-tailed t-test, p=0.3109, F(2,23)=1.230). (c,d) MeA Crfr2 KD mice did not display any changes in anxiety-like behavior compared to controls. (c) Open-field test. Left panel shows a schematic representation of the setup (n=7,8 respectively, number of rearing, two-tailed t-test, p=0.99, t=0.0127, df=13; distance- two tailed t-test, p=0.983, t=0.021, df=13). (d) Light/dark transfer test. Left panel shows a schematic representation of the setup (n=9,9 respectively, distance - two tailed t-test, p=0.693, t=0.4013, df=16; latency- two-tailed t-test, p=0.8212, t=0.229, df=16). Bars represent mean ± s.e.m.
Supplementary Figure 6 CRF-R2 neurons in the MeA express GAD65/GAD67
Double in situ hybridization of tdTomato (silver grains), and GAD65/67 mRNA (red staining) in the MeA (Bregma -1.7 mm) of the CRF-R2 tdTomato+ mouse brain. Filled arrows indicate cells coexpressing tdTomato and GAD65/67.
Supplementary Figure 7 mUcn3 administration to the MeA does not alter nonsocial exploratory behavior
(a) Exploratory behavior (number of visits to each chamber in the social approach test) of MeA saline administered mice (n=13, control) and MeA mUcn3 administered mice (n=11) did not differ (two-tailed t-test, p=0.7165, t=0.3678, df=22). (b) Average speed of MeA saline administered mice (n=13, control) and MeA mUcn3 administered mice (n=11) did not differ (two-tailed t-test, p=0.5484, t=0.6096, df=22). (c) Exploratory behavior (number of visits to each chamber in the social approach test) of MeA saline+mUcn3 administered mice (n=12, control) and MeA CRF-R2 antagonist + mUcn3 administered mice (n=8) did not differ (two-tailed t-test, p=0.5699, t=0.5788, df=18). (d) Average speed of MeA saline+mUcn3 administered mice (n=12, control) and MeA CRF-R2 antagonist+mUcn3 administered mice (n=8) did not differ (two-tailed t-test, p=0.6956, t=0.3975, df=18). Bars represent mean ± s.e.m.
Supplementary Figure 8 Optogenetic activation of MeA Ucn3 neurons does not alter nonsocial exploratory behavior
(a) Illustration of the social maze with the optogenetic setup. (b) Transgenic mice expressing Cre-recombinase specifically in Ucn3 expressing neurons (Ucn3::Cre+) were injected with AAV5-EF1α-DIO-ChR2-eYFP virus bilaterally into the MeA, resulting in site specific (MeA) and cell specific (Ucn3) expression of Channelrhodopsin-2. (c) Exploratory behavior (number of visits to each chamber in the social approach test) of Ucn3::Cre+ mice (n=3, treatment) and Ucn3::Cre− control mice (n=4) did not differ (two-tailed Mann Whitney U test, p=0.4571, U=3.5, df=6). (d) Average speed of Ucn3::Cre+ mice (n=3, control) and Ucn3::Cre− control mice (n=4) did not differ (Two-tailed Mann Whitney U test, p=0.40, U=3, df=6). Bars represent mean ± s.e.m.
Supplementary Figure 9 Mouse models of social avoidance
(a) Chronic Social Defeat - a model of environmentally-induced social avoidance where a test mouse is subjected to an aggressive bully mouse for ten consecutive days. (b) Shank3 KO, a genetic model of social avoidance and autism-like behavior.
Supplementary Figure 10 Tracking group behavior before and after DREADD-based MeA-Ucn3 inhibition
(a) Groups of four mice were introduced into the system and recorded for 12 hours each day while under low light conditions that mimic a bright night (2 lux). Each mouse was marked by a different hair dye, which enabled automatic tracking of its location. The arena consisted of an open 70×50 cm box which contained nests, feeders, water, elevated areas, and barriers. Starting from the 4th day CNO was added to the drinking water for three days, resulting in a sub-chronic inhibition of the MeA-Ucn3 cells. (b) Dyadic interactions were automatically classified based on the distance between the mice (d), and the angle of movement of one of the mice with respect to the other (θ). The algorithm used these parameters to determine if a mouse was moving toward another mouse, away from it, or neither. (c) We defined contacts as times in which the distance between the mice was less than 10 cm. We used a hidden Markov model (HMM) in order to determine the events leading up to the contact (like who initiated the contact), and how it concluded. In cases where the contact ended with one mouse following the other, we used an additional classifier to determine if the interaction was an aggressive chase-escape. (d) Functional validation of DREADD based MeA Ucn3 inhibition. cFos expression in the MeA is reduced in MeA Ucn3::Cre::hM4D injected mice following CNO administration. (e) Quantification of the cFos staining. We found significantly reduced number of cFos positive cells in the MeA of Ucn3::Cre::hM4D mice injected with CNO compared to control mice (one-tailed Mann Whitney test, p=0.0079, U=0, df=8). Bars represent mean ± s.e.m.
Supplementary Figure 11 Basal behavior in the social maze of the control groups in the different experiments
(a, b) The time WT siblings of Crfr2 KO (a) and Ucn3 KO (b) spent in each chamber of the social maze. (c, d) The time MeA-shame injected siblings of MeA Crfr2 KD (c) and MeA-sham injected siblings of LS KD mice (d) spent in each chamber of the social maze. (e) The time MeA saline administered mice spent in each chamber of the social maze. These mice were littermates of mice that were injected with mUcn3 to the MeA. (f) The time MeA saline+mUcn3 administered mice spent in each chamber of the social maze. These mice were littermates and controls to mice that were injected with mUcn3+assteressin2b (CRF-R2 antagonist). (g, h) Basal behavior in the social maze of groups served as a control for MeA Ucn3 optogenetic activation. (g) The time Ucn3::Cre− mice (light on and light off) spent in each chamber of the social maze. (h) The time Ucn3::Cre− mice (light on and light off) treated with CRF-R2 antagonist spent in each chamber of the social maze. Bars represent mean ± s.e.m.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 1725 kb)
Optogenetic activation of MeA Ucn3 neurons
MeA Ucn3-expressing neurons were optogenetically activated while the mouse was tested in the social maze. The maze comprised three arms: one with a mouse familiar to the actor, another with a novel mouse, and the third empty (non-social). The arm that contained the familiar mouse can be recognized in the movie by the note "F" below it. (MOV 5029 kb)
Tracking a freely behaving group of mice in a semi-natural environment
The video shows the tracked position of each of four mice. As mice are nocturnal, we recorded them in low light conditions that mimic a bright night (2 lux). Each mouse was marked using a different hair dye, which due to the use of sensitive cameras was distinguishable even under these light levels. The arena contained nests, feeders, water, and additional enrichments which are highlighted in the video. (MOV 11304 kb)
Aggressive chase-escape interactions
Each contact between the mice was analyzed to determine which behavior preceded it (who initiated the interaction), and how it concluded. Interactions that ended with one mouse going after the other while the other moves away were automatically classified as an aggressive chase-escape or as non-aggressive. (MOV 31834 kb)
Rights and permissions
About this article
Cite this article
Shemesh, Y., Forkosh, O., Mahn, M. et al. Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat Neurosci 19, 1489–1496 (2016). https://doi.org/10.1038/nn.4346
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4346
This article is cited by
-
Transcriptome and histological analyses on the uterus of freckle egg laying hens
BMC Genomics (2023)
-
Urocortin-3 neurons in the perifornical area are critical mediators of chronic stress on female infant-directed behavior
Molecular Psychiatry (2023)
-
A paradigm shift in translational psychiatry through rodent neuroethology
Molecular Psychiatry (2023)
-
Urocortins in the mammalian endocrine system
Acta Veterinaria Scandinavica (2019)
-
Identity domains capture individual differences from across the behavioral repertoire
Nature Neuroscience (2019)