Abstract
Limited functional recovery can be achieved through rehabilitation after incomplete spinal cord injury. Eliminating the function of a repulsive Wnt receptor, Ryk, in mice and rats by either conditional knockout in the motor cortex or monoclonal antibody infusion resulted in increased corticospinal axon collateral branches with presynaptic puncta in the spinal cord and enhanced recovery of forelimb reaching and grasping function following a cervical dorsal column lesion. Using optical stimulation, we observed that motor cortical output maps underwent massive changes after injury and that hindlimb cortical areas were recruited to control the forelimb over time. Furthermore, a greater cortical area was dedicated to controlling the forelimb in Ryk conditional knockout mice than in controls (wild-type or heterozygotes). In the absence of weekly task-specific training, recruitment of ectopic cortical areas was greatly reduced and there was no significant functional recovery even in Ryk conditional knockout mice. Our study provides evidence that maximal circuit reorganization and functional recovery can be achieved by combining molecular manipulation and targeted rehabilitation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lemon, R.N. & Griffiths, J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve 32, 261–279 (2005).
Whishaw, I.Q., Pellis, S.M., Gorny, B.P. & Pellis, V.C. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, video recording, and movement notation analysis. Behav. Brain Res. 42, 77–91 (1991).
Metz, G.A.S., Dietz, V., Schwab, M.E. & van de Meent, H. The effects of unilateral pyramidal tract section on hindlimb motor performance in the rat. Behav. Brain Res. 96, 37–46 (1998).
Schmidlin, E., Wannier, T., Bloch, J. & Rouiller, E.M. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Res. 1017, 172–183 (2004).
Weidner, N., Ner, A., Salimi, N. & Tuszynski, M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA 98, 3513–3518 (2001).
Liu, Y. et al. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 8, 1151–1159 (2005).
Liu, Y. et al. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J. Neurosci. 28, 8376–8382 (2008).
Hollis, E.R. II et al. Remodeling of spared proprioceptive circuit involving a small number of neurons supports functional recovery. Nat. Commun. 6, 6079 (2015).
Hollis, E.R. II & Zou, Y. Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proc. Natl. Acad. Sci. USA 109, 14663–14668 (2012).
Bareyre, F.M. et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 7, 269–277 (2004).
Ghosh, A. et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat. Neurosci. 13, 97–104 (2010).
Takeoka, A., Vollenweider, I., Courtine, G. & Arber, S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 159, 1626–1639 (2014).
van den Brand, R. et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 (2012).
Giger, R.J., Hollis, E.R. II & Tuszynski, M.H. Guidance molecules in axon regeneration. Cold Spring Harb. Perspect. Biol. 2, a001867 (2010).
Hollis, E.R. II. Axon guidance molecules and neural circuit remodeling after spinal cord injury. Neurotherapeutics 10.1007/s13311-015-0416-0 (2015).
Lyuksyutova, A.I. et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984–1988 (2003).
Ditunno, J.F., Little, J.W., Tessler, A. & Burns, A.S. Spinal shock revisited: a four-phase model. Spinal Cord 42, 383–395 (2004).
Leyton, A.S.F. & Sherrington, C.S. Observations on the excitable cortex of the chimpanzee, orang-utan, and gorilla. Exp. Physiol. 11, 135–222 (1917).
Streletz, L.J. et al. Transcranial magnetic stimulation: cortical motor maps in acute spinal cord injury. Brain Topogr. 7, 245–250 (1995).
Topka, H., Cohen, L.G., Cole, R.A. & Hallett, M. Reorganization of corticospinal pathways following spinal cord injury. Neurology 41, 1276–1283 (1991).
Jacobs, K.M. & Donoghue, J.P. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251, 944–947 (1991).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Ayling, O.G.S., Harrison, T.C., Boyd, J.D., Goroshkov, A. & Murphy, T.H. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat. Methods 6, 219–224 (2009).
Hira, R. et al. Transcranial optogenetic stimulation for functional mapping of the motor cortex. J. Neurosci. Methods 179, 258–263 (2009).
Kleim, J.A., Barbay, S. & Nudo, R.J. Functional reorganization of the rat motor cortex following motor skill learning. J. Neurophysiol. 80, 3321–3325 (1998).
Girgis, J. et al. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain 130, 2993–3003 (2007).
Fouad, K. & Tetzlaff, W. Rehabilitative training and plasticity following spinal cord injury. Exp. Neurol. 235, 91–99 (2012).
Schmitt, A.M. et al. Wnt-Ryk signaling mediates medial-lateral retinotectal topographic mapping. Nature 439, 31–37 (2006).
Nishimura, Y. et al. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science 318, 1150–1155 (2007).
García-Alías, G., Truong, K., Shah, P.K., Roy, R.R. & Edgerton, V.R. Plasticity of subcortical pathways promote recovery of skilled hand function in rats after corticospinal and rubrospinal tract injuries. Exp. Neurol. 266, 112–119 (2015).
Piecharka, D.M., Kleim, J.A. & Whishaw, I.Q. Limits on recovery in the corticospinal tract of the rat: partial lesions impair skilled reaching and the topographic representation of the forelimb in motor cortex. Brain Res. Bull. 66, 203–211 (2005).
Morawietz, C. & Moffat, F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch. Phys. Med. Rehabil. 94, 2297–2308 (2013).
McKenna, J.E., Prusky, G.T. & Whishaw, I.Q. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J. Comp. Neurol. 419, 286–296 (2000).
Lee, J.K. et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron 66, 663–670 (2010).
Liang, F., Rouiller, E.M. & Wiesendanger, M. Modulation of sustained electromyographic activity by single intracortical microstimuli: comparison of two forelimb motor cortical areas of the rat. Somatosens. Mot. Res. 10, 51–61 (1993).
Acknowledgements
We would like to thank Z. He, B. Zheng, F. Wang, L. Wang and the members of the Zou lab for critical reading of the manuscript, as well as comments and suggestions. This work was supported by grants to Y.Z. (RO1 NS047484, R21 NS081738, Wings for Life Foundation, and International Foundation for Research in Paraplegia).
Author information
Authors and Affiliations
Contributions
Y.Z. and E.R.H. designed the experiments. E.R.H., N.I., T.Y., C.-C.L., A.H., K.T., A.R., A.T., M.P. and E.J. performed all the experiments under the supervision of Y.Z. Y.Z. designed the antigen for the Ryk monoclonal antibody. C.-C.L. and A.R. prepared the antigen. S.-H.W. generated the hybridomas using the antigen under the supervision of A.K. A.T. and E.R.H. screened for the hybridoma and tested the function of the Ryk monoclonal antibody in vitro and in vivo.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
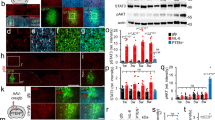
Supplementary Figure 1 Full Western blots from Figure 1c and Supplementary Figure 3a.
(a) Specificity of Ryk monoclonal antibody from Supplementary Fig. 3a. (b) Western blot of postnatal day 7 motor cortex extract from mice infected at postnatal day 0 with AAV2/1 synapsin Cre from Figure 1c. E18.5 cortex from two separate Ryk KO embryonic mouse cortices as control in right two lanes. GAPDH loading control from same blot.
Supplementary Figure 2 Sample frames from forelimb reach video.
Example at 13 weeks after C5 dorsal column lesion (1 week after C3 sham operation) demonstrates the recovery of skilled forelimb grasp. Successful reach requires the use of a grasping motion as a sweeping motion would result in the pellet being dropped in either the gap between the food pellet platform (black) and the main enclosure, or through the wire-frame floor of the enclosure.
Supplementary Figure 3 Ryk monoclonal antibody infusion in rats.
(a) Specificity of Ryk monoclonal antibody. Full-length blot presented in Supplementary Fig. 1. (b) Timeline outlining experimental details of Ryk monoclonal antibody infusion after bilateral C5 dorsal column lesion in rats.
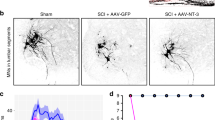
Supplementary Figure 4 Axon collateralization increased after Ryk monoclonal antibody infusion caudal to the injury.
Rats infused for 28 days with Ryk monoclonal antibody had greater levels of collateralization caudal to the lesion than control IgG infused rats (n = 6 (IgG control) 5 (Ryk monoclonal) rats, one-tailed t-test P = 0.0196 t(6) = 2.594, data presented as mean ± s.e.m.). Injury site is at 0 μm, caudal is represented with positive numbers. Axon index is thresholded pixels in sagittal spinal cord divided by thresholded pixels in transverse pyramids.
Supplementary Figure 5 Optogenetic mapping example.
Sedated mice with unilateral cranial windows were stimulated with 470nm LED by fiber optic cable to evoke muscle movements. Two examples of motor maps from one animal, pre- and 3 days post-C5 dorsal column lesion are shown.
Supplementary Figure 6 Optogenetic mapping after spinal cord injury in mice that received weekly training.
(a-b) Maps of evoked motor output in control (a) and Ryk conditional deleted mice (b) shift after spinal cord injury. Red corresponds to a larger number of mice responsive at a given location, blue to a smaller number (n = number of mice per condition/time point). (c) Proportion of motor cortex occupied by characterized motor output changes over time in response to weekly training and Ryk conditional deletion.
Supplementary Figure 7 Recovery of skilled forelimb reach for mice used in cortical mapping experiments.
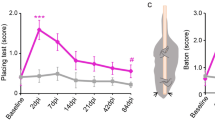
Behavioral performance on skilled forelimb reach task shows enhanced recovery after Ryk conditional deletion in contralateral motor cortex (weeks 1-8 after C5 lesion, n = 10 (control) 11 (Ryk cKO) mice, repeated measures ANOVA P=0.0304 F(1,19)=5.472). Secondary C3 eliminates enhanced recovery after Ryk conditional deletion (n = 9 (control) 8 (Ryk cKO)), while unilateral pyramidotomy (n = 8 (control) 7 (Ryk cKO)) completely ablates the ability of mice to perform the task. Data presented as mean±s.e.m.
Supplementary Figure 8 Optogenetic mapping after spinal cord injury without weekly training.
(a-b) Maps of evoked motor output in control (a) and Ryk conditional deleted mice (b) shift after spinal cord injury. Red corresponds to a larger number of mice responsive at a given location, blue to a smaller number. (c) Proportions of motor cortex occupied by characterized motor output are relatively stable in the absence of training after injury.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 6259 kb)
Rights and permissions
About this article
Cite this article
Hollis, E., Ishiko, N., Yu, T. et al. Ryk controls remapping of motor cortex during functional recovery after spinal cord injury. Nat Neurosci 19, 697–705 (2016). https://doi.org/10.1038/nn.4282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4282
This article is cited by
-
Regulation of axonal regeneration after mammalian spinal cord injury
Nature Reviews Molecular Cell Biology (2023)
-
Effects of biological sex mismatch on neural progenitor cell transplantation for spinal cord injury in mice
Nature Communications (2022)
-
Natural and targeted circuit reorganization after spinal cord injury
Nature Neuroscience (2022)
-
Restoring After Central Nervous System Injuries: Neural Mechanisms and Translational Applications of Motor Recovery
Neuroscience Bulletin (2022)
-
Frizzled 1 and Wnt1 as new potential therapeutic targets in the traumatically injured spinal cord
Cellular and Molecular Life Sciences (2020)