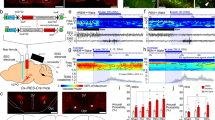
Abstract
Do sedatives engage natural sleep pathways? It is usually assumed that anesthetic-induced sedation and loss of righting reflex (LORR) arise by influencing the same circuitry to lesser or greater extents. For the α2 adrenergic receptor agonist dexmedetomidine, we found that sedation and LORR were in fact distinct states, requiring different brain areas: the preoptic hypothalamic area and locus coeruleus (LC), respectively. Selective knockdown of α2A adrenergic receptors from the LC abolished dexmedetomidine-induced LORR, but not sedation. Instead, we found that dexmedetomidine-induced sedation resembled the deep recovery sleep that follows sleep deprivation. We used TetTag pharmacogenetics in mice to functionally mark neurons activated in the preoptic hypothalamus during dexmedetomidine-induced sedation or recovery sleep. The neuronal ensembles could then be selectively reactivated. In both cases, non-rapid eye movement sleep, with the accompanying drop in body temperature, was recapitulated. Thus, α2 adrenergic receptor–induced sedation and recovery sleep share hypothalamic circuitry sufficient for producing these behavioral states.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Franks, N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 (2008).
Rihel, J. & Schier, A.F. Sites of action of sleep and wake drugs: insights from model organisms. Curr. Opin. Neurobiol. 23, 831–840 (2013).
Adams, R. et al. Efficacy of dexmedetomidine compared with midazolam for sedation in adult intensive care patients: a systematic review. Br. J. Anaesth. 111, 703–710 (2013).
Bol, C.J.J.G., Danhof, M., Stanski, D.R. & Mandema, J.W. Pharmacokinetic-pharmacodynamic characterization of the cardiovascular, hypnotic, EEG and ventilatory responses to dexmedetomidine in the rat. J. Pharmacol. Exp. Ther. 283, 1051–1058 (1997).
Seidel, W.F., Maze, M., Dement, W.C. & Edgar, D.M. Alpha-2 adrenergic modulation of sleep: time-of-day-dependent pharmacodynamic profiles of dexmedetomidine and clonidine in the rat. J. Pharmacol. Exp. Ther. 275, 263–273 (1995).
MacDonald, E., Scheinin, M., Scheinin, H. & Virtanen, R. Comparison of the behavioral and neurochemical effects of the two optical enantiomers of medetomidine, a selective alpha-2-adrenoceptor agonist. J. Pharmacol. Exp. Ther. 259, 848–854 (1991).
Sanders, R.D. & Maze, M. Noradrenergic trespass in anesthetic and sedative states. Anesthesiology 117, 945–947 (2012).
Nelson, L.E. et al. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 98, 428–436 (2003).
Lakhlani, P.P. et al. Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc. Natl. Acad. Sci. USA 94, 9950–9955 (1997).
Aghajanian, G.K. & VanderMaelen, C.P. α2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science 215, 1394–1396 (1982).
Correa-Sales, C., Rabin, B.C. & Maze, M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology 76, 948–952 (1992).
Takahashi, K., Kayama, Y., Lin, J.S. & Sakai, K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience 169, 1115–1126 (2010).
Berridge, C.W., Schmeichel, B.E. & Espana, R.A. Noradrenergic modulation of wakefulness/arousal. Sleep Med. Rev. 16, 187–197 (2012).
Carter, M.E., de Lecea, L. & Adamantidis, A. Functional wiring of hypocretin and LC-NE neurons: implications for arousal. Front. Behav. Neurosci. 7, 43 (2013).
Carter, M.E. et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010).
Gilsbach, R. et al. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Mol. Pharmacol. 75, 1160–1170 (2009).
Hu, F.Y. et al. Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine beta-hydroxylase knockout mice. Anesthesiology 117, 1006–1017 (2012).
Szymusiak, R., Gvilia, I. & McGinty, D. Hypothalamic control of sleep. Sleep Med. 8, 291–301 (2007).
Alam, M.A., Kumar, S., McGinty, D., Alam, M.N. & Szymusiak, R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J. Neurophysiol. 111, 287–299 (2014).
Takahashi, K., Lin, J.S. & Sakai, K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience 161, 269–292 (2009).
Sherin, J.E., Shiromani, P.J., McCarley, R.W. & Saper, C.B. Activation of ventrolateral preoptic neurons during sleep. Science 271, 216–219 (1996).
Lu, J., Greco, M.A., Shiromani, P. & Saper, C.B. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 20, 3830–3842 (2000).
Saper, C.B., Fuller, P.M., Pedersen, N.P., Lu, J. & Scammell, T.E. Sleep state switching. Neuron 68, 1023–1042 (2010).
Reijmers, L.G., Perkins, B.L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Garner, A.R. et al. Generation of a synthetic memory trace. Science 335, 1513–1516 (2012).
Reijmers, L. & Mayford, M. Genetic control of active neural circuits. Front. Mol. Neurosci. 2, 27 (2009).
Alexander, G.M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein–coupled receptors. Neuron 63, 27–39 (2009).
Stegmeier, F., Hu, G., Rickles, R.J., Hannon, G.J. & Elledge, S.J. A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 102, 13212–13217 (2005).
Zecharia, A.Y. et al. The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J. Neurosci. 29, 2177–2187 (2009).
Tan, C.M., Wilson, M.H., MacMillan, L.B., Kobilka, B.K. & Limbird, L.E. Heterozygous alpha 2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc. Natl. Acad. Sci. USA 99, 12471–12476 (2002).
Gong, H. et al. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J. Physiol. (Lond.) 556, 935–946 (2004).
Hunter, J.C. et al. Assessment of the role of alpha2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br. J. Pharmacol. 122, 1339–1344 (1997).
Tong, Q., Ye, C.P., Jones, J.E., Elmquist, J.K. & Lowell, B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000 (2008).
Drew, G.M., Gower, A.J. & Marriott, A.S. Alpha 2-adrenoceptors mediate clonidine-induced sedation in the rat. Br. J. Pharmacol. 67, 133–141 (1979).
McGregor, R. & Siegel, J.M. Illuminating the locus coeruleus: control of posture and arousal. Nat. Neurosci. 13, 1448–1449 (2010).
McGinty, D.J. & Sterman, M.B. Sleep suppression after basal forebrain lesions in the cat. Science 160, 1253–1255 (1968).
Sterman, M.B. & Clemente, C.D. Forebrain inhibitory mechanisms: sleep patterns induced by basal forebrain stimulation in the behaving cat. Exp. Neurol. 6, 103–117 (1962).
Sherin, J.E., Elmquist, J.K., Torrealba, F. & Saper, C.B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 18, 4705–4721 (1998).
Saito, Y.C. et al. GABAergic neurons in the preoptic area send direct inhibitory projections to orexin neurons. Front. Neural Circuits 7, 192 (2013).
Gallopin, T. et al. Identification of sleep-promoting neurons in vitro. Nature 404, 992–995 (2000).
Modirrousta, M., Mainville, L. & Jones, B.E. Gabaergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience 129, 803–810 (2004).
Brown, R.E., Basheer, R., McKenna, J.T., Strecker, R.E. & McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 92, 1087–1187 (2012).
Gelegen, C. et al. Staying awake—a genetic region that hinders alpha adrenergic receptor agonist-induced sleep. Eur. J. Neurosci. 40, 2311–2319 (2014).
Murray, A.J. et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat. Neurosci. 14, 297–299 (2011).
Tang, W. et al. Faithful expression of multiple proteins via 2A-peptide self-processing: a versatile and reliable method for manipulating brain circuits. J. Neurosci. 29, 8621–8629 (2009).
Krashes, M.J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Klugmann, M. et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol. Cell. Neurosci. 28, 347–360 (2005).
Mastakov, M.Y., Baer, K., Xu, R., Fitzsimons, H. & During, M.J. Combined injection of rAAV with mannitol enhances gene expression in the rat brain. Mo. Ther. 3, 225–232 (2001).
Vyssotski, A.L. et al. EEG responses to visual landmarks in flying pigeons. Curr. Biol. 19, 1159–1166 (2009).
Gerashchenko, D. et al. Identification of a population of sleep-active cerebral cortex neurons. Proc. Natl. Acad. Sci. USA 105, 10227–10232 (2008).
Acknowledgements
This work was supported by the Medical Research Council (G0901892, N.P.F., S.G.B. and W.W.; G0800399, W.W.), the Biotechnology and Biological Sciences Research Council (BBSRC) (G021691 and BB/K018159/1, N.P.F., S.G.B. and W.W.), the Wellcome Trust (WT094211MA, S.G.B., N.P.F. and W.W.), a BBSRC CASE studentship (E.A.S.), a Wellcome Trust Vacation Scholarship (Z.E.P.), a BBSRC doctoral training grant (BB/F017324/1, E.C.H.), UK-China Scholarships for Excellence/China Scholarship scheme (X.Y. and Z.Y.) and the ERASMUS program (İ.G. and A.M.).
Author information
Authors and Affiliations
Contributions
N.P.F. and W.W. conceived and designed the experiments. Z.Z., V.F., İ.G., A.M., E.A.S., Z.Y., A.Y.Z., X.Y., R.Y., Z.E.P. and E.C.H. performed the experiments. A.L.V. provided the Neurologgers. N.P.F., W.W. and S.G.B. contributed to the data analysis. N.P.F. and W.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
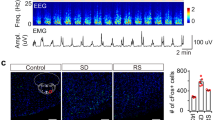
Supplementary Figure 1 Dexmedetomidine-induced sedation and recovery sleep induce cfos expression in overlapping regions of the mouse hypothalamic preoptic area and septum.
(a) Schematic of the preoptic hypothalamic and septal area: left-hand drawing, coronal section, boxed areas, magnified on the right, show the relevant anatomical sites corresponding to the photographs below; (b) Photographs of nuclear cfos protein (DAB staining method) 30 minutes after saline, 30 minutes and 1 hour after dexmedetomidine (100 μg kg−1) injections or 2 hours into recovery sleep. Abbreviations, LPO, lateral preoptic area; LSV, lateral septum, ventral; MPO, medial preoptic area SHy, septo-hypothalamic nucleus; STLD, stria terminalis lateral dorsal; STMA, stria terminalis medial anterior; VLPO, ventral lateral preoptic area.
Supplementary Figure 2 Induction of hM3Dq–mCHERRY transgene during recovery sleep in MnPO-TetTag-hM3Dq mice.
The TetTag AAVs were injected at the midline into the MnPO area. The photographs show coronal sections stained for hM3Dq–mCHERRY expression (red). The left-hand photograph in the top row shows basal transgene expression with no doxycycline; the middle picture shows induced hM3Dq–mCHERRY transgene expression in the MnPO area 2 hours into recovery sleep; the third figure shows expression 4 days later with the mice back on doxycycline. The images below show the low expression 4 weeks later and the figure to the right shows the relatively low hM3Dq–mCHERRY transgene induction following dexmedetomidine sedation. Scale bar, 50 μm; Abbreviations: LPO, lateral preoptic area; MnPO, median preoptic area; 3V, third ventricle.
Supplementary Figure 3 LPO TetTag expression patterns: Induction of hM3Dq-mCHERRY expression 2 hours following dexmedetomidine-induced sedation where AAVs were bilaterally injected into the LPO and surrounding areas.
Each letter, A through to L, summarizes the expression in individual mice as seen by serial coronal sectioning through the AAV injection sites. Red indicates hM3Dq-mCHERRY expression. The boxed sections in animals I-L were mice where hM3Dq-mCHERRY transgene was induced but there was no behavioral or EEG signs of sedation. Some LPO-TetTag-hM3Dq mice that had been sedated with dexmedetomidine did not show any subsequent CNO-induced behavior at either the EEG or behavioral levels relative to that observed with saline injection. Although the hM3Dq-mCHERRY gene was clearly induced by dexmedetomidine treatment in these animals, the transgene expression sites were on the lateral margin of the LPO area or even further out laterally (animals I, J, K and L). Thus activating these lateral TetTagged neurons with CNO was not sufficient to induce sleep. One animal, (mouse I), had induced hM3Dq receptor only in the BST areas, but also exhibited no CNO-induced behavior, so BST stimulation alone was not sufficient to recapitulate dexmedetomidine-induced sedation. Abbreviations: BST, bed nucleus stria terminalis; LPO, lateral preoptic area; MPO, medial preoptic area; VLPO, ventral lateral preoptic area.
Supplementary Figure 4 MnPO TetTag expression patterns: Induction of hM3Dq-mCHERRY expression 2 hours into recovery sleep after sleep deprivation (animals A-F) or two hours following dexmedetomidine-induced sedation (animals G-I) where AAVs were midline-injected into MnPO and surrounding areas.
Each letter, A through to I, summarizes the expression in individual mice as seen by serial coronal sectioning through the AAV injection site. Red indicates induced hM3Dq-mCHERRY expression. The boxed sections, J-L, were from mice where the hM3Dq-mCHERRY transgene was induced following sleep-deprivation and recovery sleep, but there was no behavioral or EEG signs of NREM sleep following CNO administration. In animals K & L, for example, the intended midline injection of AAV into MnPO missed, and resulted in unilateral hM3Dq-mCHERRY induction in the LPO area; but activating these receptors with CNO was insufficient to trigger sleep behavior. Abbreviations: BST, bed nucleus stria terminalis; LPO, lateral preoptic area; MPO, medial preoptic area; VLPO, ventral lateral preoptic area.
Supplementary Figure 5 CNO induces nuclear cfos expression (green) in hM3Dq-mCHERRY (red) expressing neurons in the LPO area of LPO-TetTag-hM3Dq mice.
Double-label immunocytochemistry with antisera to mCHERRY and cfos. The hM3Dq-mCHERRY expression was induced by a sedative dose (100 μg kg−1) of dexmedetomidine and then mice were injected with CNO and their brains taken 2 hours afterwards. Arrows indicate examples of co-labeled cells.
Supplementary Figure 6 TetTagged neurons are excited by CNO and are predominantly GABAergic.
(a) A representative example of the effects of 5 μM CNO on a mCHERRY-positive neuron in LPO-TetTag-hM3Dq mice. On average, neurons (n=8; 3 mice) were depolarized by 10.2 ± 2.1 mV. In the example shown, action potential firing was triggered. (b) These neurons were predominantly GABAergic. 84% were gad1 and/ or gad2 positive, as assayed by single-cell qPCR. The remainder were glutamatergic (vglut2-positive). (c) A representative example of the qPCR assay run out on a gel from three of the neurons. LAD, sizes shown are base pairs.
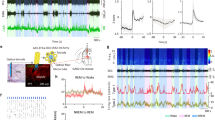
Supplementary Figure 7 Characteristics of natural NREM sleep and circadian body temperature of virally-injected C57BL/6 mice, the strain used for the TetTagging experiments.
(a) Percentage of NREM sleep throughout the 24-hour sleep-wake cycle (n=10). (b) Fourier Transform power spectra when the EEG and EMG signals from the natural sleep-wake cycle were scored as either sleep (red) or wake (black). Each spectrum is calculated by combining EEG segments totally 20 minutes. The envelopes represent the s.e.m. The inserts show representative EEG traces, and the accompanying calibration bars represent 100 μV and 500 msec (n=10). (c) Body temperature throughout the normal 24-hour sleep-wake cycle. The envelopes represent the s.e.m. All of these data are for LPO-TetTag-hM3Dq (n=10) and MnPO-TetTag-hM3Dq mice (n=10) combined, because these were indistinguishable. All data are from 12 hours light: 12 hours dark cycles.
Supplementary Figure 8 SC-TetTag-hM3Dq (negative control) mice
(a), schematic illustrating bilateral injection of the two TetTag-DREADD AAVs (AAV-Pcfos-tTA and AAV-PTRE-tight-hM3Dq-mCHERRY) into the superior colliculi and the experimental procedures with drug administration and time plan following that outlined in Fig. 3b. (b) Four days following either 100 μg kg−1 dexmedetomidine-induced sedation or after 2 hours into recovery sleep following sleep deprivation, CNO was administered to SC-TetTag-hM3Dq mice. Open circles (n=6): control CNO injection without prior sedation or recovery sleep. Filled red circles (n=5): CNO injection after prior dexmedetomidine sedation. Filled red triangles (n=5): CNO injection after prior recovery sleep. The mice injected with CNO following dexmedetomidine sedation (two-way ANOVA, P=0.79) or recovery sleep (two-way ANOVA, P=0.71) were indistinguishable from controls.
Supplementary Figure 9 Knock down of adrenergic α2A receptor transcripts in the preoptic area (LPO) of the hypothalamus had no effect on dexmedetomidine-induced sedation.
(a) Schematic illustrating bilateral injection of AAVs expressing either dsRED-mir30-shadra2a or dsRED-mir30-shscramble transgenes into the LPO of adult mice. (b) The total distance travelled in 15 minutes by saline-injected (white bars) or dexmedetomidine-injected (100 μg kg−1) (red bars) mice was not significantly different (n=6 scramble, n=7 shRNA; P=0.19) for mice with AAVs expressing either the knock-down or scramble transgenes. The boxes represent the s.e.m, and the bars show the range of the data.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Text (PDF 1370 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Ferretti, V., Güntan, İ. et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat Neurosci 18, 553–561 (2015). https://doi.org/10.1038/nn.3957
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3957
This article is cited by
-
Biphasic Npas4 expression promotes inhibitory plasticity and suppression of fear memory consolidation in mice
Molecular Psychiatry (2024)
-
Dexmedetomidine modulates neuronal activity of horizontal limbs of diagonal band via α2 adrenergic receptor in mice
BMC Anesthesiology (2023)
-
Somatostatin neurons in prefrontal cortex initiate sleep-preparatory behavior and sleep via the preoptic and lateral hypothalamus
Nature Neuroscience (2023)
-
Neuro-orchestration of sleep and wakefulness
Nature Neuroscience (2023)
-
Emergence of consciousness from anesthesia through ubiquitin degradation of KCC2 in the ventral posteromedial nucleus of the thalamus
Nature Neuroscience (2023)