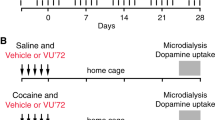
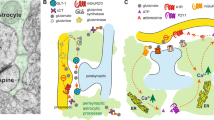
Abstract
Relapse to cocaine use necessitates remodeling excitatory synapses in the nucleus accumbens and synaptic reorganization requires matrix metalloproteinase (MMP) degradation of the extracellular matrix proteins. We found enduring increases in MMP-2 activity in rats after withdrawal from self-administered cocaine and transient increases in MMP-9 during cue-induced cocaine relapse. Cue-induced heroin and nicotine relapse increased MMP activity, and increased MMP activity was required for both cocaine relapse and relapse-associated synaptic plasticity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vocci, F. & Ling, W. Pharmacol. Ther. 108, 94–108 (2005).
Russo, S.J. et al. Trends Neurosci. 33, 267–276 (2010).
Conrad, K.L. et al. Nature 454, 118–121 (2008).
Anderson, S.M. et al. Nat. Neurosci. 11, 344–353 (2008).
Gipson, C.D. et al. Neuron 77, 867–872 (2013).
Huntley, G.W. Nat. Rev. Neurosci. 13, 743–757 (2012).
Sternlicht, M.D. & Werb, Z. Annu. Rev. Cell Dev. Biol. 17, 463–516 (2001).
Cingolani, L.A. et al. Neuron 58, 749–762 (2008).
Bozdagi, O., Nagy, V., Kwei, K.T. & Huntley, G.W. J. Neurophysiol. 98, 334–344 (2007).
Levin, J.I. Bioorg. Med. Chem. Lett. 11, 2189–2192 (2001).
Verslegers, M., Lemmens, K., Van Hove, I. & Moons, L. Prog. Neurobiol. 105, 60–78 (2013).
Wolf, M.E. & Ferrario, C.R. Neurosci. Biobehav. Rev. 35, 185–211 (2010).
Moussawi, K. et al. Nat. Neurosci. 12, 182–189 (2009).
Robinson, T.E. & Kolb, B. Neuropharmacology 47 (suppl. 1), 33–46 (2004).
Mash, D. et al. PLoS ONE 2, 1187 (2007).
Kovatsi, L. et al. Toxicol. Mech. Methods 23, 377–381 (2013).
Samochowiec, A. et al. Brain Res. 1327, 103–106 (2010).
Brown, T.E. et al. Learn. Mem. 14, 214–223 (2007).
Van den Oover, M. et al. Neuropsychopharmacology 35, 2120–2133 (2010).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Elsevier Academic Press, Burlington, 2007).
Levin, J.I. et al. Bioorg. Med. Chem. Lett. 11, 2189–2192 (2001).
Shen, H., Moussawi, K., Zhou, W., Toda, S. & Kalivas, P.W. Proc. Natl. Acad. Sci. USA 108, 19407–19412 (2011).
Gipson, C.D. et al. Proc. Natl. Acad. Sci. USA 110, 9124–9129 (2013).
Kupai, K. et al. J. Pharmacol. Toxicol. Methods 61, 205–209 (2010).
Liu, W., Furuichi, T., Miyake, M., Rosenberg, G.A. & Liu, K.J. J. Neurosci. Res. 85, 829–836 (2007).
Morales-Mulia, M., de Gortari, P., Amaya, M.I. & Mendez, M. J. Mol. Neuroscience. 49, 289–300 (2013).
Shen, H., Sesack, S.R., Toda, S. & Kalivas, P.W. Brain Struct. Funct. 213, 149–157 (2008).
Acknowledgements
This research was support by US National Institutes of Health grants DA003906, DA012513 and DA015369.
Author information
Authors and Affiliations
Contributions
A.C.W.S. conducted the behavioral and zymography studies, analyzed data and wrote the manuscript. Y.M.K. conducted the electrophysiological experiments. M.D.S. conducted the western blotting and contributed to the spine analysis. C.D.G. contributed to spine analysis. A.W. developed the in vivo zymography assay to generate preliminary data for this study. C.A.T. performed surgeries and conducted behavioral experiments. P.W.K. oversaw the project, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Control FITC-gelatin substrate experiments show linearity of fluorescence over time after injection.
The catabolism of the FITC-gelatin substrate shows linear increases in gelatinolytic fluorescence over 15 - 60 minutes incubation in vivo. Injections were made into the dorsal hippocampus due to relatively higher constitutive MMP activity compared with the NAcore or striatum. N= 3 at each time point.
Supplementary Figure 2 Lever pressing during self-administration and extinction of cocaine, nicotine and heroin.
a) Active and inactive lever pressing data for animals that were used for in vitro measurements of zymography, Western blotting, electrophysiology or dendrite morphology in figures 1 and 2. b) Training data for rats used in cocaine studies in figure 2e,f. c) Training data for rats used in sucrose studies in figure 2g.
Supplementary Figure 3 Lack of increased MMP-2 and MMP-9 activity in the dorsal striatum or accumbens shell following cue-induced reinstatement of cocaine seeking.
There was no change in gelatinolytic fluorescence in either the dorsal striatum or the nucleus accumbens shell between yoked-saline controls and animals that underwent 15 minutes of cue-induced reinstatement. This indicates anatomical specificity for MMP-dependent plasticity within within the striatum is largely confined to the NAcore. Dashed lines on the micrographs encompass the injection site that was masked-out for quantification. Unpaired Student’s t-test revealed no significant effect of reinstatement on fluorescence, dorsal striatum t(4) = 1.411, p > 0.05, NAshell t(4) = 0.2112, p > 0.05.
Supplementary Figure 4 There were no changes in MMP-2 protein or mRNA of each MMP-2, MMP-9 or TIMP-2.
a) MMP-2 protein was quantified in NAcore tissue obtained from yoked saline, cocaine extinguished and after 15 min of cued reinstatement in cocaine-trained rats. There was no difference between treatment groups using a one-way ANOVA. b) mRNA content quantified by PCR. Paired Student’s t-tests did not reveal any significant differences between groups. MMP-2 t(10) = 0.6321, p > .05, MMP-9 t(10) = 0.934, p > .05, TIMP-2 t(10) = 0.814m p > .05. S = Yoked Saline, Reinst = Reinstated
Supplementary Figure 5 MMP inhibition did not have an effect on NMDA decay time or sEPSCs.
a) Representative traces showing full length AMPA and NMDA current recordings. b) No effect of MMP inhibition on NMDA decay time. One-way ANOVA F(8,67) = 0.83, p > .05. c) MMP inhibition did not alter sEPSCs amplitude in any condition. One-way ANOVA F(8,67) = 1.34, p > .05. d) MMP inhibition did not alter sEPSCs frequency in any condition. One-way ANOVA F(8,67) = 0.88, p > .05. N is shown in bars as number of neurons recorded (panels a, d, e) or animals quantified (panels b, c) with each animal being the average of 6-12 neurons.
Supplementary Figure 6 Representative images and dendritic spine density frequency plot.
a,b) For dendritic spine analysis, images of entire neurons were taken at 1 µm resolution, and then 45-55 µm segments located between 75-200 µm from the soma and after the first branch point were imaged at 0.1 µm resolution for 3-dimensional reconstruction and morphological analysis. The yellow rectangle indicates the location of the dendritic segment that was imaged from these neurons. c) Representative micrographs of quantified dendritic segments from each group. d) Shows cumulative frequency distribution for dendritic spine density. There was a noticeable shift to the right in extinguished cocaine treated animals relative to yoked saline indicating greater spine density in cocaine-extinguished rats (see statistical analysis of mean values in figure 5). While MMP-2 inhibition reversed this effect in cocaine-extinguished subjects, MMP-9 inhibition was ineffective. Reinstatement did not alter the distribution relative to extinguished vehicle treatment, and MMP-2, but not MMP-9 inhibition returned the reinstated cocaine distribution to yoked saline distribution.
Supplementary Figure 7 MMP inhibition did not affect synaptic strength in yoked saline animals.
MMP inhibition did not affect synaptic strength in yoked saline animals. a) Shows A/N following vehicle, MMP-2 or MMP-9 inhibition in yoked-saline controls. One-way ANOVA revealed F(2,19)= 3.41, p > .05. b) Neither MMP-2 nor MMP-9 inhibition affected spine head diameter in yoked-saline controls. One-way ANOVA revealed F(2,9) = 1.01, p > .05. c) Neither MMP-2 nor MMP-9 inhibition affected spine density in yoked-saline controls. One-way ANOVA F(2,9) = 0.12, p < .05.
Supplementary Figure 8 Histological verification of microinjection sites for animals microinjected with vehicle or MMP inhibitors prior to reinstatement in Figure 2.
Rats with injection cannula outside of the NAcore were excluded from behavioral analysis.
Supplementary Figure 9 Full-length western blots corresponding to truncated blots shown in Figure 1h.
ERY pattern of spotting the gel repeated across the gel. E- extinguished, R- reinstated, Y- yoked saline
Supplementary Figure 10 Lever pressing following vehicle microinjection in dose-response analysis in Figure 2 was stable across three reinstatement trials.
The experiments in figures 2e-f were conducted as a within-subject crossover design consisting of 3 reinstatement trials per animal (unless a microinjection cannula became clogged, in which case only 2 trials were conducted. These data show the response to vehicle when it was randomly given in the first, second or third trial, and that there is no difference in vehicle reinstatement across 3 trials. The data argue against the possibility that the data if figure 6 were influenced by the order of drug injection across trials. One-Way ANOVA revealed F(2, 22) = 0.02, p > .05.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1359 kb)
Supplementary Methods Checklist
(PDF 398 kb)
Source data
Rights and permissions
About this article
Cite this article
Smith, A., Kupchik, Y., Scofield, M. et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci 17, 1655–1657 (2014). https://doi.org/10.1038/nn.3846
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3846
This article is cited by
-
Neuronal TIMP2 regulates hippocampus-dependent plasticity and extracellular matrix complexity
Molecular Psychiatry (2023)
-
Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens
Molecular Psychiatry (2022)
-
Activation of mesocorticolimbic dopamine projections initiates cue-induced reinstatement of reward seeking in mice
Acta Pharmacologica Sinica (2022)
-
Interactions of neuroimmune signaling and glutamate plasticity in addiction
Journal of Neuroinflammation (2021)
-
Drug addiction: from bench to bedside
Translational Psychiatry (2021)