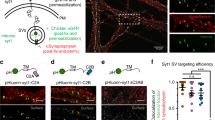
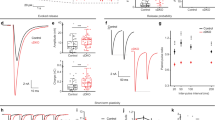
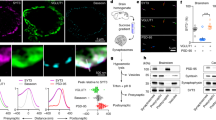
Abstract
The Ca2+ sensor for rapid synaptic vesicle exocytosis, synaptotagmin 1 (syt), is largely composed of two Ca2+-sensing C2 domains, C2A and C2B. We investigated the apparent synergy between the tandem C2 domains by altering the length and rigidity of the linker that connects them. The behavior of the linker mutants revealed a correlation between the ability of the C2 domains to penetrate membranes in response to Ca2+ and to drive evoked neurotransmitter release in cultured mouse neurons, uncovering a step in excitation-secretion coupling. Using atomic force microscopy, we found that the synergy between these C2 domains involved intra-molecular interactions between them. Thus, syt function is markedly affected by changes in the physical nature of the linker that connects its tandem C2 domains. Moreover, the linker mutations uncoupled syt-mediated regulation of evoked and spontaneous release, revealing that syt also acts as a fusion clamp before the Ca2+ trigger.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lu, T. & Trussell, L.O. Inhibitory transmission mediated by asynchronous transmitter release. Neuron 26, 683–694 (2000).
Iremonger, K.J. & Bains, J.S. Integration of asynchronously released quanta prolongs the postsynaptic spike window. J. Neurosci. 27, 6684–6691 (2007).
Lau, P.M. & Bi, G.Q. Synaptic mechanisms of persistent reverberatory activity in neuronal networks. Proc. Natl. Acad. Sci. USA 102, 10333–10338 (2005).
Kavalali, E.T. et al. Spontaneous neurotransmission: an independent pathway for neuronal signaling? Physiology (Bethesda) 26, 45–53 (2011).
Sutton, M.A. et al. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125, 785–799 (2006).
Söllner, T. et al. SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324 (1993).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 (1998).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Chapman, E.R. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77, 615–641 (2008).
Sutton, R.B., Ernst, J.A. & Brunger, A.T. Crystal structure of the cytosolic C2A–C2B domains of synaptotagmin III. Implications for Ca(+2)-independent snare complex interaction. J. Cell Biol. 147, 589–598 (1999).
Fuson, K.L., Montes, M., Robert, J.J. & Sutton, R.B. Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association. Biochemistry 46, 13041–13048 (2007).
Chapman, E.R. & Davis, A.F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998).
Bai, J., Tucker, W.C. & Chapman, E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Chicka, M.C., Hui, E.F., Liu, H.S. & Chapman, E.R. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca(2+). Nat. Struct. Mol. Biol. 15, 827–835 (2008).
Martens, S., Kozlov, M.M. & McMahon, H.T. How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 (2007).
Hui, E., Johnson, C.P., Yao, J., Dunning, F.M. & Chapman, E.R. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell 138, 709–721 (2009).
Bhalla, A., Chicka, M.C., Tucker, W.C. & Chapman, E.R. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13, 323–330 (2006).
Hui, E. et al. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat. Struct. Mol. Biol. 18, 813–821 (2011).
Yao, J., Gaffaney, J.D., Kwon, S.E. & Chapman, E.R. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell 147, 666–677 (2011).
Groffen, A.J. et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 327, 1614–1618 (2010).
Xu, J., Pang, Z.P.P., Shin, O.H. & Südhof, T.C. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat. Neurosci. 12, 759–766 (2009).
Kochubey, O. & Schneggenburger, R. Synaptotagmin increases the dynamic range of synapses by driving Ca(2)+-evoked release and by clamping a near-linear remaining Ca(2)+ sensor. Neuron 69, 736–748 (2011).
Vyleta, N.P. & Smith, S.M. Spontaneous glutamate release is independent of calcium influx and tonically activated by the calcium-sensing receptor. J. Neurosci. 31, 4593–4606 (2011).
Liu, H., Dean, C., Arthur, C.P., Dong, M. & Chapman, E.R. Autapses and networks of hippocampal neurons exhibit distinct synaptic transmission phenotypes in the absence of synaptotagmin I. J. Neurosci. 29, 7395–7403 (2009).
Craxton, M. Synaptotagmin gene content of the sequenced genomes. BMC Genomics 5, 43 (2004).
Kwon, O.J., Gainer, H., Wray, S. & Chin, H. Identification of a novel protein containing two C2 domains selectively expressed in the rat brain and kidney. FEBS Lett. 378, 135–139 (1996).
Bhalla, A., Chicka, M.C. & Chapman, E.R. Analysis of the synaptotagmin family during reconstituted membrane fusion. Uncovering a class of inhibitory isoforms. J. Biol. Chem. 283, 21799–21807 (2008).
Gaffaney, J.D., Dunning, F.M., Wang, Z., Hui, E. & Chapman, E.R. Synaptotagmin C2B domain regulates Ca2+-triggered fusion in vitro: critical residues revealed by scanning alanine mutagenesis. J. Biol. Chem. 283, 31763–31775 (2008).
Hui, E., Bai, J. & Chapman, E.R. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys. J. 91, 1767–1777 (2006).
Fernandez, I. et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069 (2001).
Ubach, J., Zhang, X., Shao, X., Südhof, T.C. & Rizo, J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 17, 3921–3930 (1998).
Bai, J., Wang, P. & Chapman, E.R. C2A activates a cryptic Ca(2+)-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA 99, 1665–1670 (2002).
Araç, D. et al. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13, 209–217 (2006).
Choi, U.B. et al. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat. Struct. Mol. Biol. 17, 318–324 (2010).
Huang, H. & Cafiso, D.S. Conformation and membrane position of the region linking the two C2 domains in synaptotagmin 1 by site-directed spin labeling. Biochemistry 47, 12380–12388 (2008).
Doose, S., Neuweiler, H., Barsch, H. & Sauer, M. Probing polyproline structure and dynamics by photoinduced electron transfer provides evidence for deviations from a regular polyproline type II helix. Proc. Natl. Acad. Sci. USA 104, 17400–17405 (2007).
Voets, T. et al. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc. Natl. Acad. Sci. USA 98, 11680–11685 (2001).
Bhalla, A., Tucker, W.C. & Chapman, E.R. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+ and Sr2+ concentration to SNARE-mediated membrane fusion. Mol. Biol. Cell 16, 4755–4764 (2005).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Zhang, X., Kim-Miller, M.J., Fukuda, M., Kowalchyk, J.A. & Martin, T.F.J. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34, 599–611 (2002).
Bai, J., Wang, C.T., Richards, D.A., Jackson, M.B. & Chapman, E.R. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron 41, 929–942 (2004).
Zhang, Z., Hui, E., Chapman, E.R. & Jackson, M.B. Regulation of exocytosis and fusion pores by synaptotagmin-effector interactions. Mol. Biol. Cell 21, 2821–2831 (2010).
Kozlov, M.M. & Chernomordik, L.V. The protein coat in membrane fusion: lessons from fission. Traffic 3, 256–267 (2002).
Sun, S. et al. Receptor binding enables botulinum neurotoxin B to sense low pH for translocation channel assembly. Cell Host Microbe 10, 237–247 (2011).
Koh, T.W. & Bellen, H.J. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 26, 413–422 (2003).
Paddock, B.E. et al. Membrane penetration by synaptotagmin is required for coupling calcium binding to vesicle fusion in vivo. J. Neurosci. 31, 2248–2257 (2011).
Xue, M. et al. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila complexins orchestrates synaptic vesicle exocytosis. Neuron 64, 367–380 (2009).
Finn, R.D. et al. The Pfam protein families database. Nucleic Acids Res. 38, D211–D222 (2010).
Bansal, D. & Campbell, K.P. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol. 14, 206–213 (2004).
Perin, M.S., Fried, V.A., Mignery, G.A., Jahn, R. & Südhof, T.C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 345, 260–263 (1990).
Desai, R.C. et al. The C2B domain of synaptotagmin is a Ca(2+)-sensing module essential for exocytosis. J. Cell Biol. 150, 1125–1136 (2000).
Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K. & Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain-reaction. Gene 77, 51–59 (1989).
Dean, C. et al. Axonal and dendritic synaptotagmin isoforms revealed by a pHluorin-syt functional screen. Mol. Biol. Cell 23, 1715–1727 (2012).
Kwon, S.E. & Chapman, E.R. Glycosylation is dispensable for sorting of synaptotagmin 1 but is critical for targeting of SV2 and synaptophysin to recycling synaptic vesicles. J. Biol. Chem. 287, 35658–35668 (2012).
Efron, B. & Tibshirani, R. An Introduction to the Bootstrap (Chapman & Hall, New York, 1993).
Xu, J., Mashimo, T. & Südhof, T.C. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 54, 567–581 (2007).
Acknowledgements
We thank D. Ruhl for help with bootstrap analysis, and we thank S. Butcher and J. Burke for help with the SAXS measurements. H.T. was supported by a Newton International Fellowship. This study was supported by a grant from the US National Institutes of Health (MH 61876). E.R.C. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
H.L. and R.X. performed and analyzed the electrophysiological experiments. H.B. performed and analyzed the biochemical experiments. H.T. and J.M.E. performed and analyzed the AFM measurements. E.R.C. designed experiments and analyzed the data. H.L., H.B., J.M.E. and E.R.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 The experiments in Fig. 2a, b, d and 3a, b were repeated at 2 mM [Ca2+]o.
(a) Representative eEPSC traces recorded from WT, syt KO, and KO neurons that expressed each linker mutant. (b, c) Scatter plots of single eEPSC amplitudes (b) and latencies to peak (c). (d) Representative eEPSCs recorded in paired-pulse experiments with 50 ms inter-stimulus intervals. (e) Scatter plot of the PPRs, calculated as in Fig. 3. For each condition, we recorded from 26–29 cells from a total of six coverslips, where two coverslips were obtained from each of three independent litters of mice. The mean values ± SEM are indicated. Bootstrap statistical analysis was carried out, and the results are provided in Supplementary Table 1. Asterisks indicate differences compared with WT; *** p < 0.001.
Supplementary Figure 2 Re-expression of syt fully rescues normal synaptic transmission in syt KO neurons.
(a) Representative eEPSC traces recorded from WT neurons, and syt KO neurons that expressed WT syt cDNA, in the presence of 10 or 2 mM [Ca2+]o. (b) Scatter plots of the individual eEPSC amplitudes. For each condition, we recorded from 26 cells from a total of six coverslips, where two coverslips were obtained from each of three independent litters of mice. The mean values ± SEM are indicated. P-values, determined using a bootstrap approach, are indicated.
Supplementary Figure 3 Syt linker mutants rescue the size of the RRP in syt KO neurons.
(a) Example EPSCs in response to a pulse of hypertonic sucrose (500 mM) for 8 s. (b) Scatter plot of the total transfer charge upon exposure to sucrose; all of the syt linker mutants fully rescued the diminished size of the RRP in the KO. For each condition we recorded from 9–12 neurons from a total of three coverslips; each coverslip was derived from an independent litter of mice. The mean values ± SEM are indicated. Statistical significance was determined using a bootstrap approach; no significant differences among neurons expressing each of the linker mutants, only the KO had alterations in the RRP; detailed analysis is provided in Supplementary Table 1. Asterisks indicate differences compared with WT; *** p < 0.001.
Supplementary Figure 4 Correlation plots of syt linker mutant t-SNARE or PS binding activity versus evoked transmission.
(a) t-SNARE binding activity (defined as the mole of syt bound per mole of syntaxin (syx)) of WT or linker mutant cytoplasmic domains (C2AB) was measured using a co-flotation assay. The final [Ca2+]free was 1 mM; [EGTA] was 0.2 mM. Four independent experiments were carried out. (b) t-SNARE binding activity of WT or linker mutant forms of C2AB were plotted versus eEPSC amplitude from Fig. 2b. (c) Binding of WT or linker mutant forms of C2AB to PS-bearing liposomes was monitored using a co-sedimentation assay; depletion of the supernatant was monitored via SDS-PAGE and staining with Coomassie blue (the staining intensity reflects free C2AB in the supernatant), and these data were used to calculate the amount of bound material, in the absence (–; 0.2 mM EGTA) or presence (+) of 1 mM [Ca2+]free. Three independent experiments were carried out. (d) PS binding activity (defined as the apparent Kd for lipid; note that [lipid] and not [liposome] values were used, thus resulting in apparent Kd values in the mM range) of WT or linker mutants were plotted versus the eEPSC values that were, again, from Fig. 2b; error bars represent SEM. Equations and p-values for the linear regressions are listed in Supplementary Table 1. For clarity, the upper and lower portions of each gel (i.e. regions lacking proteins), were cropped.
Supplementary Figure 5 Positive correlation between the membrane penetration activity of WT and mutant forms of syt and eEPSC amplitude at a lower concentration of Ca2+.
(a–b) Scatter plots of peak values of the normalized NBD fluorescence of C2A*(3)-C2B (a) and C2A-C2B*(b) in 100 μM Ca2+, using liposomes that harbored 25% PS. For each condition, 3 independent experiments were carried out. (c, d). Positive correlation between the membrane penetration activity of C2A (c), and C2B (d), and eEPSC amplitude recorded at 2 mM [Ca2+]o, taken from Supplemental Fig. 1b. The mean values ± SEM are indicated. Statistical significance was determined using a bootstrap approach. Asterisks indicate differences compared with WT; * p < 0.05, *** p < 0.001. Detailed statistical analysis, and the equations and p-values for the linear regressions, are provided in Supplementary Table 1.
Supplementary Figure 6 Partial correlation between the PS binding and membrane penetration activity of linker mutant forms of syt.
Penetration data from Fig. 4 were plotted versus the apparent affinities for binding membranes from Supplementary Fig. 4d; penetration data for C2A and C2B are shown in panel a and b, respectively; error bars represent SEM. Equations and p-values for the linear regressions are provided in Supplementary Table 1.
Supplementary Figure 7 Correlation between membrane penetration activity and the ability of syt linker mutants to drive Ca2+-triggered lipid mixing in a reconstituted membrane fusion assay.
(a) A schematic diagram of the reconstituted fusion system; the donor (NBD) fluorescence increases, due to dequenching, upon fusion. (b, c) Representative traces of reconstituted membrane fusion reactions regulated by WT or linker mutant forms of syt; the arrow indicates the addition of Ca2+ (1 mM free). (d) Scatter plot of the extent of fusion 1 h after addition of Ca2+. For each condition, four independent experiments were carried out. (e, f) Positive correlation between membrane penetration of loop 3 in either C2A or C2B, taken from Fig. 4c,f, with the extent of fusion. Plotted values represent the mean ± SEM. Statistical significance was determined using a bootstrap approach. Asterisks indicate differences compared with WT; * p < 0.05, ** p < 0.01. Detailed statistical analysis, and equations and p-values for the linear regressions, are provided in Supplementary Table 1.
Supplementary Figure 8 WT C2AB adopts a horseshoe shaped structure.
(a) Schematic representations of the horseshoe structures in the AFM images shown in Fig. 5a. (b) Illustration of the method used to determine the lengths of horseshoe structures and extended blobs.
Supplementary Figure 9 FRET measurements revealed that the intramolecular interaction between C2A and C2B is not influenced by either Ca2+ or membranes.
(a) Representative emission spectra of AEDANS-labeled C2AB in 0.1 mM EGTA, 0.1 or 1 mM free Ca2+ with, or without, liposomes that harbored 25% PS; the labeled protein was excited at 295 nm. Peak 1 is the emission peak of two native Trp residues in C2B domain. Peak 2 is the emission peak of AEDANS conjugated to the C2A domain. As a control, unlabeled C2AB was analyzed in parallel (in EGTA, minus liposomes). (b) Scatter plot of peak 2 divided by peak 1, under each condition; plotted values represent the mean ± SEM. For each condition, 4–6 independent experiments were carried out. Statistical significance was determined using a bootstrap approach; no significant differences were observed (Supplementary Table 1).
Supplementary Figure 10 Correlation analysis of mEPSC frequency.
(a) Lack of correlation between the ability of the syt linker mutants to regulate evoked versus spontaneous release. Data for mEPSCs and eEPSCs are from Fig. 6d and Fig. 2b, respectively. (b) Correlation plot of the ability of the syt linker mutants to suppress spontaneous release, and to bind t-SNAREs in the absence of Ca2+. Data for mEPSCs are from Fig. 6d and Ca2+-independent t-SNARE binding activity was obtained from Supplementary Fig. 4a. Plotted values represent the mean ± SEM. Equations and p-values for the linear regressions are listed in Supplementary Table 1.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 17284 kb)
Source data
Rights and permissions
About this article
Cite this article
Liu, H., Bai, H., Xue, R. et al. Linker mutations reveal the complexity of synaptotagmin 1 action during synaptic transmission. Nat Neurosci 17, 670–677 (2014). https://doi.org/10.1038/nn.3681
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3681
This article is cited by
-
Synaptotagmin-7 outperforms synaptotagmin-1 to promote the formation of large, stable fusion pores via robust membrane penetration
Nature Communications (2023)
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
The complexin C-terminal amphipathic helix stabilizes the fusion pore open state by sculpting membranes
Nature Structural & Molecular Biology (2022)
-
Resolving kinetic intermediates during the regulated assembly and disassembly of fusion pores
Nature Communications (2020)
-
Synaptotagmin-1 enables frequency coding by suppressing asynchronous release in a temperature dependent manner
Scientific Reports (2019)