Abstract
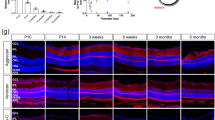
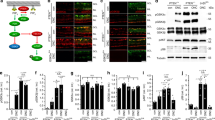
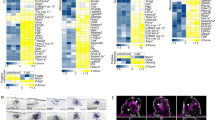
In the adult mammalian CNS, chondroitin sulfate proteoglycans (CSPGs) and myelin-associated inhibitors (MAIs) stabilize neuronal structure and restrict compensatory sprouting following injury. The Nogo receptor family members NgR1 and NgR2 bind to MAIs and have been implicated in neuronal inhibition. We found that NgR1 and NgR3 bind with high affinity to the glycosaminoglycan moiety of proteoglycans and participate in CSPG inhibition in cultured neurons. Nogo receptor triple mutants (Ngr1−/−; Ngr2−/−; Ngr3−/−; which are also known as Rtn4r, Rtn4rl2 and Rtn4rl1, respectively), but not single mutants, showed enhanced axonal regeneration following retro-orbital optic nerve crush injury. The combined loss of Ngr1 and Ngr3 (Ngr1−/−; Ngr3−/−), but not Ngr1 and Ngr2 (Ngr1−/−; Ngr2−/−), was sufficient to mimic the triple mutant regeneration phenotype. Regeneration in Ngr1−/−; Ngr3−/− mice was further enhanced by simultaneous ablation of Rptpσ (also known as Ptprs), a known CSPG receptor. Collectively, our results identify NgR1 and NgR3 as CSPG receptors, suggest that there is functional redundancy among CSPG receptors, and provide evidence for shared mechanisms of MAI and CSPG inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Park, K.K. et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966 (2008).
Liu, B.P., Cafferty, W.B., Budel, S.O. & Strittmatter, S.M. Extracellular regulators of axonal growth in the adult central nervous system. Phil. Trans. R. Soc. Lond. B. 361, 1593–1610 (2006).
Winzeler, A.M. et al. The lipid sulfatide is a novel myelin-associated inhibitor of CNS axon outgrowth. J. Neurosci. 31, 6481–6492 (2011).
Silver, J. & Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 (2004).
Bregman, B.S. et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378, 498–501 (1995).
Li, S. et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 24, 10511–10520 (2004).
Bradbury, E.J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
García-Alías, G., Barkhuysen, S., Buckle, M. & Fawcett, J.W. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 12, 1145–1151 (2009).
Massey, J.M. et al. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J. Neurosci. 26, 4406–4414 (2006).
Atwal, J.K. et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322, 967–970 (2008).
Fournier, A.E., GrandPre, T. & Strittmatter, S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346 (2001).
Venkatesh, K. et al. The Nogo-66 receptor homolog NgR2 is a sialic acid–dependent receptor selective for myelin-associated glycoprotein. J. Neurosci. 25, 808–822 (2005).
Chivatakarn, O., Kaneko, S., He, Z., Tessier-Lavigne, M. & Giger, R.J. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J. Neurosci. 27, 7117–7124 (2007).
Kim, J.E., Liu, B.P., Park, J.H. & Strittmatter, S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 44, 439–451 (2004).
Zheng, B. et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl. Acad. Sci. USA 102, 1205–1210 (2005).
Sivasankaran, R. et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat. Neurosci. 7, 261–268 (2004).
Schweigreiter, R. et al. Versican V2 and the central inhibitory domain of Nogo-A inhibit neurite growth via p75NTR/NgR-independent pathways that converge at RhoA. Mol. Cell. Neurosci. 27, 163–174 (2004).
Kantor, D.B. et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44, 961–975 (2004).
Rhodes, K.E. & Fawcett, J.W. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J. Anat. 204, 33–48 (2004).
McKeon, R.J., Hoke, A. & Silver, J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp. Neurol. 136, 32–43 (1995).
Pizzorusso, T. et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 (2002).
Houle, J.D. et al. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J. Neurosci. 26, 7405–7415 (2006).
Powell, E.M., Mercado, M.L., Calle-Patino, Y. & Geller, H.M. Protein kinase C mediates neurite guidance at an astrocyte boundary. Glia 33, 288–297 (2001).
Shen, Y. et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596 (2009).
Aricescu, A.R., McKinnell, I.W., Halfter, W. & Stoker, A.W. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol. Cell. Biol. 22, 1881–1892 (2002).
Fry, E.J., Chagnon, M.J., Lopez-Vales, R., Tremblay, M.L. & David, S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma–deficient mice. Glia 58, 423–433 (2010).
Sapieha, P.S. et al. Receptor protein tyrosine phosphatase sigma inhibits axon regrowth in the adult injured CNS. Mol. Cell. Neurosci. 28, 625–635 (2005).
Lee, J.K. et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG- and OMgp-deficient mice. Neuron 66, 663–670 (2010).
Cafferty, W.B., Duffy, P., Huebner, E. & Strittmatter, S.M. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J. Neurosci. 30, 6825–6837 (2010).
Yamashita, T., Fujitani, M., Yamagishi, S., Hata, K. & Mimura, F. Multiple signals regulate axon regeneration through the Nogo receptor complex. Mol. Neurobiol. 32, 105–111 (2005).
Williams, G. et al. Ganglioside inhibition of neurite outgrowth requires Nogo receptor function: identification of interaction sites and development of novel antagonists. J. Biol. Chem. 283, 16641–16652 (2008).
Barton, W.A. et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J. 22, 3291–3302 (2003).
Zhang, L., Kuang, X. & Zhang, J. Nogo receptor 3, a paralog of Nogo-66 receptor 1 (NgR1), may function as a NgR1 co-receptor for Nogo-66. J. Genet. Genomics 38, 515–523 (2011).
Ohlsson, M., Mattsson, P. & Svensson, M. A temporal study of axonal degeneration and glial scar formation following a standardized crush injury of the optic nerve in the adult rat. Restor. Neurol. Neurosci. 22, 1–10 (2004).
Yin, Y. et al. Oncomodulin links inflammation to optic nerve regeneration. Proc. Natl. Acad. Sci. USA 106, 19587–19592 (2009).
Leibinger, M. et al. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci. 29, 14334–14341 (2009).
Fisher, D. et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J. Neurosci. 31, 14051–14066 (2011).
Fischer, D., He, Z. & Benowitz, L.I. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J. Neurosci. 24, 1646–1651 (2004).
Fischer, D., Petkova, V., Thanos, S. & Benowitz, L.I. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J. Neurosci. 24, 8726–8740 (2004).
Kadoya, K. et al. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron 64, 165–172 (2009).
Fujita, Y., Endo, S., Takai, T. & Yamashita, T. Myelin suppresses axon regeneration by PIR-B/SHP–mediated inhibition of Trk activity. EMBO J. 30, 1389–1401 (2011).
Niederöst, B.P., Zimmermann, D.R., Schwab, M.E. & Bandtlow, C.E. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J. Neurosci. 19, 8979–8989 (1999).
Shypitsyna, A., Malaga-Trillo, E., Reuter, A. & Stuermer, C.A. Origin of Nogo-A by domain shuffling in an early jawed vertebrate. Mol. Biol. Evol. 28, 1363–1370 (2010).
McGee, A.W., Yang, Y., Fischer, Q.S., Daw, N.W. & Strittmatter, S.M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309, 2222–2226 (2005).
Lee, H. et al. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J. Neurosci. 28, 2753–2765 (2008).
Zagrebelsky, M., Schweigreiter, R., Bandtlow, C.E., Schwab, M.E. & Korte, M. Nogo-A stabilizes the architecture of hippocampal neurons. J. Neurosci. 30, 13220–13234 (2010).
Raiker, S.J. et al. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J. Neurosci. 30, 12432–12445 (2010).
Karlén, A. et al. Nogo receptor 1 regulates formation of lasting memories. Proc. Natl. Acad. Sci. USA 106, 20476–20481 (2009).
Briani, C., Berger, J.S. & Latov, N. Antibodies to chondroitin sulfate C: a new detection assay and correlations with neurological diseases. J. Neuroimmunol. 84, 117–121 (1998).
Robak, L.A. et al. Molecular basis of the interactions of the Nogo-66 receptor and its homolog NgR2 with myelin-associated glycoprotein: development of NgROMNI-Fc, a novel antagonist of CNS myelin inhibition. J. Neurosci. 29, 5768–5783 (2009).
Acknowledgements
We thank M. Tremblay for Rptpσ−/− mice, M. Greenberg for Ngr3−/− mice, B. Pierchala for p75NTR−/− mice, B. Bates, D. Howland and M.L. Mercado for their assistance in the generation and initial analysis of Ngr1−/−; Ngr2−/−; Ngr3−/− mice, U. Rutishauser for Endo-N, D. Figge and Y. Yasui for assistance in ELISA binding assays, Y. Yin for training in optic nerve surgery, Y. Duan for generation of the RPTPσ(Ig1–3)-Fc construct, J. Barbieri for technical assistance and M.M. Zaleska for project administration. This work was supported by Neuroscience Training Grant T32EY017878 and the University of Michigan Rackham Merit Fellowship (T.L.D.), Cellular and Molecular Biology Training Grant T32GM007315 (K.T.B. and Y.A.M.), National Research Service Award Ruth Kirschstein Fellowship F31NS061589 (S.J.R.), the New York State Spinal Cord Injury Research Program, the Dr. Miriam and Sheldon G. Adelson Medical Foundation on Neural Repair and Rehabilitation, the US Department of Veterans Affairs (1I01RX000229-01), the National Institute of Neurological Disorders and Stroke (R56NS047333, R.J.G.) and the National Eye Institute (L.I.B.).
Author information
Authors and Affiliations
Contributions
R.J.G. conceived the study. T.L.D., L.I.B., H.M.G. and R.J.G. designed the experiments. T.L.D., K.T.B., Y.A.M., Y. Koriyama, S.J.R., C.D.L., Y. Katagiri and R.J.G. performed the experiments. T.L.D., K.T.B. and Y. Koriyama contributed to data analysis and figure preparation. K.L.A., A.W., C.G.G. and B.Z. generated and provided mice or reagents for the study, T.L.D. and R.J.G. wrote the manuscript. All of the authors read and agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 1797 kb)
Rights and permissions
About this article
Cite this article
Dickendesher, T., Baldwin, K., Mironova, Y. et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 15, 703–712 (2012). https://doi.org/10.1038/nn.3070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3070
This article is cited by
-
Inhibition of the Nogo-pathway in experimental spinal cord injury: a meta-analysis of 76 experimental treatments
Scientific Reports (2023)
-
Lateral olfactory tract usher substance (LOTUS), an endogenous Nogo receptor antagonist, ameliorates disease progression in amyotrophic lateral sclerosis model mice
Cell Death Discovery (2023)
-
Zinc-a2-Glycoprotein Acts as a Component of PNN to Protect Hippocampal Neurons from Apoptosis
Molecular Neurobiology (2023)
-
Nogo-B promotes invasion and metastasis of nasopharyngeal carcinoma via RhoA-SRF-MRTFA pathway
Cell Death & Disease (2022)
-
Extracellular Matrix in Neural Plasticity and Regeneration
Cellular and Molecular Neurobiology (2022)