Abstract
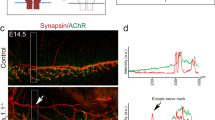
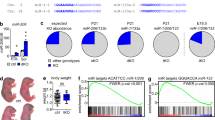
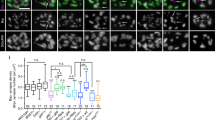
Developing skeletal myofibers in vertebrates are intrinsically 'pre-patterned' for motor nerve innervation. However, the intrinsic factors that regulate muscle pre-patterning remain unknown. We found that a functional skeletal muscle dihydropyridine receptor (DHPR, the L-type Ca2+ channel in muscle) was required for muscle pre-patterning during the development of the neuromuscular junction (NMJ). Targeted deletion of the β1 subunit of DHPR (Cacnb1) in mice led to muscle pre-patterning defects, aberrant innervation and precocious maturation of the NMJ. Reintroducing Cacnb1 into Cacnb1−/− muscles reversed the pre-patterning defects and restored normal development of the NMJ. The mechanism by which DHPRs govern muscle pre-patterning is independent of their role in excitation-contraction coupling, but requires Ca2+ influx through the L-type Ca2+ channel. Our findings indicate that the skeletal muscle DHPR retrogradely regulates the patterning and formation of the NMJ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hall, Z.W. & Sanes, J.R. Synaptic structure and development: the neuromuscular junction. Cell 72 Suppl, 99–121 (1993).
Lin, W. et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410, 1057–1064 (2001).
Yang, X., Li, W., Prescott, E.D., Burden, S.J. & Wang, J.C. DNA topoisomerase IIbeta and neural development. Science 287, 131–134 (2000).
Yang, X. et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 30, 399–410 (2001).
Kim, N. & Burden, S.J. MuSK controls where motor axons grow and form synapses. Nat. Neurosci. 11, 19–27 (2008).
Flanagan-Steet, H., Fox, M.A., Meyer, D. & Sanes, J.R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 132, 4471–4481 (2005).
Panzer, J.A., Song, Y. & Balice-Gordon, R.J. In vivo imaging of preferential motor axon outgrowth to and synaptogenesis at prepatterned acetylcholine receptor clusters in embryonic zebrafish skeletal muscle. J. Neurosci. 26, 934–947 (2006).
Liu, Y. et al. Essential roles of the acetylcholine receptor gamma-subunit in neuromuscular synaptic patterning. Development 135, 1957–1967 (2008).
Misgeld, T., Kummer, T.T., Lichtman, J.W. & Sanes, J.R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. USA 102, 11088–11093 (2005).
Lin, W. et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 46, 569–579 (2005).
Fischbach, G.D., Nameroff, M. & Nelson, P.G. Electrical properties of chick skeletal muscle fibers developing in cell culture. J. Cell. Physiol. 78, 289–299 (1971).
Cohen, S.A. & Fischbach, G.D. Regulation of muscle acetylcholine sensitivity by muscle activity in cell culture. Science 181, 76–78 (1973).
Spector, I. & Prives, J.M. Development of electrophysiological and biochemical membrane properties during differentiation of embryonic skeletal muscle in culture. Proc. Natl. Acad. Sci. USA 74, 5166–5170 (1977).
Franzini-Armstrong, C. & Jorgensen, A.O. Structure and development of E-C coupling units in skeletal muscle. Annu. Rev. Physiol. 56, 509–534 (1994).
Tsien, R.W., Ellinor, P.T. & Horne, W.A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol. Sci. 12, 349–354 (1991).
Gregg, R.G. et al. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation-contraction coupling. Proc. Natl. Acad. Sci. USA 93, 13961–13966 (1996).
Takeshima, H. et al. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine receptor gene. Nature 369, 556–559 (1994).
Nakai, J. et al. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380, 72–75 (1996).
Barone, V. et al. Contractile impairment and structural alterations of skeletal muscles from knockout mice lacking type 1 and type 3 ryanodine receptors. FEBS Lett. 422, 160–164 (1998).
Sanes, J.R. & Lichtman, J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2, 791–805 (2001).
Arikkath, J. & Campbell, K.P. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 13, 298–307 (2003).
Ball, S.L. et al. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest. Ophthalmol. Vis. Sci. 43, 1595–1603 (2002).
Beurg, M. et al. Recovery of Ca2+ current, charge movements, and Ca2+ transients in myotubes deficient in dihydropyridine receptor beta 1 subunit transfected with beta 1 cDNA. Biophys. J. 73, 807–818 (1997).
Coronado, R., Morrissette, J., Sukhareva, M. & Vaughan, D.M. Structure and function of ryanodine receptors. Am. J. Physiol. 266, C1485–C1504 (1994).
Bertocchini, F. et al. Requirement for the ryanodine receptor type 3 for efficient contraction in neonatal skeletal muscles. EMBO J. 16, 6956–6963 (1997).
Duclert, A. & Changeux, J.P. Acetylcholine receptor gene expression at the developing neuromuscular junction. Physiol. Rev. 75, 339–368 (1995).
Blau, H.M., Chiu, C.P. & Webster, C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell 32, 1171–1180 (1983).
Lee, K.S. & Tsien, R.W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature 302, 790–794 (1983).
Ahern, C.A. et al. Ca2+ current and charge movements in skeletal myotubes promoted by the beta-subunit of the dihydropyridine receptor in the absence of ryanodine receptor type 1. Biophys. J. 84, 942–959 (2003).
Strube, C., Beurg, M., Powers, P.A., Gregg, R.G. & Coronado, R. Reduced Ca2+ current, charge movement, and absence of Ca2+ transients in skeletal muscle deficient in dihydropyridine receptor beta 1 subunit. Biophys. J. 71, 2531–2543 (1996).
Jorgensen, A.O., Shen, A.C., Arnold, W., Leung, A.T. & Campbell, K.P. Subcellular distribution of the 1,4-dihydropyridine receptor in rabbit skeletal muscle in situ: an immunofluorescence and immunocolloidal gold-labeling study. J. Cell Biol. 109, 135–147 (1989).
Gomez-Ospina, N., Tsuruta, F., Barreto-Chang, O., Hu, L. & Dolmetsch, R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell 127, 591–606 (2006).
Schroder, E., Byse, M. & Satin, J. L-type calcium channel C terminus autoregulates transcription. Circ. Res. 104, 1373–1381 (2009).
Zhang, Y. et al. The beta subunit of voltage-gated Ca2+ channels interacts with and regulates the activity of a novel isoform of Pax6. J. Biol. Chem. 285, 2527–2536 (2010).
Pai, A.C. Developmental genetics of a lethal mutation, muscular dysgenesis (mdg), in the mouse. I. Genetic analysis and gross morphology. Dev. Biol. 11, 82–92 (1965).
Pai, A.C. Developmental genetics of a lethal mutation, muscular dysgenesis (mdg), in the mouse. II. Developmental analysis. Dev. Biol. 11, 93–109 (1965).
Powell, J.A., Rieger, F., Blondet, B., Dreyfus, P. & Pincon-Raymond, M. Distribution and quantification of ACh receptors and innervation in diaphragm muscle of normal and mdg mouse embryos. Dev. Biol. 101, 168–180 (1984).
Knudson, C.M. et al. Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J. Biol. Chem. 264, 1345–1348 (1989).
Koenig, J., Bournaud, R., Powell, J.A. & Rieger, F. Appearance of contractile activity in muscular dysgenesis (mdg/mdg) mouse myotubes during coculture with normal spinal cord cells. Dev. Biol. 92, 188–196 (1982).
Rieger, F. et al. Restoration of dysgenic muscle contraction and calcium channel function by co-culture with normal spinal cord neurons. Nature 330, 563–566 (1987).
Lomo, T. & Rosenthal, J. Control of ACh sensitivity by muscle activity in the rat. J. Physiol. (Lond.) 221, 493–513 (1972).
Witzemann, V., Brenner, H.R. & Sakmann, B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J. Cell Biol. 114, 125–141 (1991).
Valenzuela, D.M. et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 15, 573–584 (1995).
Lomo, T. & Westgaard, R.H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J. Physiol. (Lond.) 252, 603–626 (1975).
Méjat, A. et al. Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat. Neurosci. 8, 313–321 (2005).
Tang, H. et al. A histone deacetylase 4/myogenin positive feedback loop coordinates denervation-dependent gene induction and suppression. Mol. Biol. Cell 20, 1120–1131 (2009).
Rotzler, S., Schramek, H. & Brenner, H.R. Metabolic stabilization of endplate acetylcholine receptors regulated by Ca2+ influx associated with muscle activity. Nature 349, 337–339 (1991).
Deisseroth, K., Mermelstein, P.G., Xia, H. & Tsien, R.W. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr. Opin. Neurobiol. 13, 354–365 (2003).
West, A.E., Griffith, E.C. & Greenberg, M.E. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 3, 921–931 (2002).
Arber, S. et al. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23, 659–674 (1999).
Acknowledgements
We thank J. Takahashi, E. Olson, B. Szaro, J. Johnson, C. Green, K. Walton, J. Terman, J. McArdle and R. Bassel-Duby for critical reading and commenting on the manuscript, S. Cannon, D. Francis, E. Kavalali and I. Bezprozvanny for valuable suggestions on muscle physiology and S. Burden for AChRα and MuSK in situ probes. This study was supported by grants (to W.L.) from the US National Institutes of Health/National Institute of Neurological Disorders and Stroke (NS 055028), the Edward Mallinckrodt, Jr. Scholar Program, and the Cain Foundation in Medical Research at University of Texas Southwestern Medical Center, and by a grant (to P.D.A.) from the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (P01AR044750).
Author information
Authors and Affiliations
Contributions
F.C., Y.L. and Y.S. carried out the experiment and collected and analyzed the data. P.D.A. provided the RyR mutant mice. R.G.G. provided the DHPR mutant mice. W.L. supervised the project and wrote the manuscript with critical input from F.C., Y.L., Y.S., P.D.A. and R.G.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 1505 kb)
Rights and permissions
About this article
Cite this article
Chen, F., Liu, Y., Sugiura, Y. et al. Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nat Neurosci 14, 570–577 (2011). https://doi.org/10.1038/nn.2792
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2792
This article is cited by
-
A genome-wide scan to identify signatures of selection in two Iranian indigenous chicken ecotypes
Genetics Selection Evolution (2021)
-
Skeletal muscle CaV1.1 channelopathies
Pflügers Archiv - European Journal of Physiology (2020)
-
Discovery and characterization of functional modules associated with body weight in broilers
Scientific Reports (2019)
-
Postsynaptic CaV1.1-driven calcium signaling coordinates presynaptic differentiation at the developing neuromuscular junction
Scientific Reports (2019)
-
The Ca2+ influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance
Nature Communications (2017)