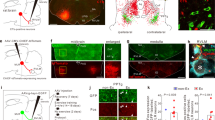
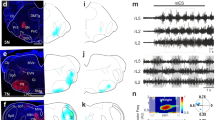
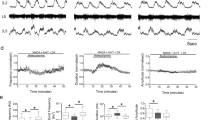
Abstract
The brainstem locomotor system is believed to be organized serially from the mesencephalic locomotor region (MLR) to reticulospinal neurons, which in turn project to locomotor neurons in the spinal cord. We identified brainstem muscarinoceptive neurons in lampreys (Petromyzon marinus) that received parallel inputs from the MLR and projected back to reticulospinal cells to amplify and extend the duration of locomotor output. These cells responded to muscarine with extended periods of excitation, received direct muscarinic excitation from the MLR and projected glutamatergic excitation to reticulospinal neurons. Targeted blockade of muscarine receptors over these neurons profoundly reduced MLR-induced excitation of reticulospinal neurons and markedly slowed MLR-evoked locomotion. The presence of these neurons forces us to rethink the organization of supraspinal locomotor control, to include a sustained feedforward loop that boosts locomotor output.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Grillner, S. Ion channels and locomotion. Science 278, 1087–1088 (1997).
Grillner, S., Wallén, P., Saitoh, K., Kozlov, A. & Robertson, B. Neural bases of goal-directed locomotion in vertebrates—an overview. Brain Res. Rev. 57, 2–12 (2008).
Dubuc, R. et al. Initiation of locomotion in lampreys. Brain Res. Rev. 57, 172–182 (2008).
Armstrong, D.M. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog. Neurobiol. 26, 273–361 (1986).
Shik, M.L., Severin, F.V. & Orlovskii, G.N. [Control of walking and running by means of electric stimulation of the midbrain.]. Biofizika 11, 659–666 (1966).
Skinner, R.D. & Garcia-Rill, E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 323, 385–389 (1984).
Bernau, N.A., Puzdrowski, R.L. & Leonard, R.B. Identification of the midbrain locomotor region and its relation to descending locomotor pathways in the Atlantic stingray, Dasyatis sabina. Brain Res. 557, 83–94 (1991).
Cabelguen, J.M., Bourcier-Lucas, C. & Dubuc, R. Bimodal locomotion elicited by electrical stimulation of the midbrain in the salamander Notophthalmus viridescens. J. Neurosci. 23, 2434–2439 (2003).
Deliagina, T.G., Zelenin, P.V. & Orlovsky, G.N. Encoding and decoding of reticulospinal commands. Brain Res. Brain Res. Rev. 40, 166–177 (2002).
Sirota, M.G., Di Prisco, G.V. & Dubuc, R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur. J. Neurosci. 12, 4081–4092 (2000).
Brocard, F. & Dubuc, R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J. Neurophysiol. 90, 1714–1727 (2003).
Le Ray, D. et al. Nicotinic activation of reticulospinal cells involved in the control of swimming in lampreys. Eur. J. Neurosci. 17, 137–148 (2003).
Brocard, F. et al. The transformation of a unilateral locomotor command into a symmetrical bilateral activation in the brainstem. J. Neurosci. 30, 523–533 (2010).
Jahn, K. et al. Supraspinal locomotor control in quadrupeds and humans. Prog. Brain Res. 171, 353–362 (2008).
Sholomenko, G.N., Funk, G.D. & Steeves, J.D. Avian locomotion activated by brainstem infusion of neurotransmitter agonists and antagonists. I. Acetylcholine excitatory amino acids and substance P. Exp. Brain Res. 85, 659–673 (1991).
Homma, Y., Skinner, R.D. & Garcia-Rill, E. Effects of pedunculopontine nucleus (PPN) stimulation on caudal pontine reticular formation (PnC) neurons in vitro. J. Neurophysiol. 87, 3033–3047 (2002).
Smetana, R.W., Alford, S. & Dubuc, R. Muscarinic receptor activation elicits sustained, recurring depolarizations in reticulospinal neurons. J. Neurophysiol. 97, 3181–3192 (2007).
Rovainen, C.M. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells. J. Neurophysiol. 30, 1000–1023 (1967).
Garcia-Rill, E. & Skinner, R.D. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 411, 13–20 (1987).
Rye, D.B., Lee, H.J., Saper, C.B. & Wainer, B.H. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J. Comp. Neurol. 269, 315–341 (1988).
Pombal, M.A., Marin, O. & Gonzalez, A. Distribution of choline acetyltransferase-immunoreactive structures in the lamprey brain. J. Comp. Neurol. 431, 105–126 (2001).
Di Prisco, G.V., Pearlstein, E., Robitaille, R. & Dubuc, R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science 278, 1122–1125 (1997).
Garcia-Rill, E., Skinner, R.D., Conrad, C., Mosley, D. & Campbell, C. Projections of the mesencephalic locomotor region in the rat. Brain Res. Bull. 17, 33–40 (1986).
Steeves, J.D. & Jordan, L.M. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res. 307, 263–276 (1984).
Orlovsky, G.N. Connexions of the reticulo-spinal neuronswith the “locomotor regions” in the brainstem. Biofizika 1, 171–177 (1970).
Jahn, K. et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage 39, 786–792 (2008).
Curró Dossi, R., Pare, D. & Steriade, M. Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. J. Neurophysiol. 65, 393–406 (1991).
Egorov, A.V., Hamam, B.N., Fransen, E., Hasselmo, M.E. & Alonso, A.A. Graded persistent activity in entorhinal cortex neurons. Nature 420, 173–178 (2002).
Klink, R. & Alonso, A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J. Neurophysiol. 77, 1829–1843 (1997).
Dickson, C.T. & Alonso, A. Muscarinic induction of synchronous population activity in the entorhinal cortex. J. Neurosci. 17, 6729–6744 (1997).
Dickson, C.T. et al. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J. Neurophysiol. 83, 2562–2579 (2000).
Tahvildari, B., Alonso, A.A. & Bourque, C.W. Ionic basis of ON and OFF persistent activity in layer III lateral entorhinal cortical principal neurons. J. Neurophysiol. 99, 2006–2011 (2008).
Garcia-Rill, E. & Skinner, R.D. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 411, 1–12 (1987).
Mamiya, K., Bay, K., Skinner, R.D. & Garcia-Rill, E. Induction of long-lasting depolarization in medioventral medulla neurons by cholinergic input from the pedunculopontine nucleus. J. Appl. Physiol. 99, 1127–1137 (2005).
McClellan, A.D., McPherson, D. & O'Donovan, M.J. Combined retrograde labeling and calcium imaging in spinal cord and brainstem neurons of the lamprey. Brain Res. 663, 61–68 (1994).
Abramoff, M.D., Magelhaes, P.J. & Ram, S.J. Image processing with ImageJ. Biophotonics Int. 11, 36–42 (2004).
Blanton, M.G., Lo Turco, J.J. & Kriegstein, A.R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J. Neurosci. Methods 30, 203–210 (1989).
Acknowledgements
We thank D. Veilleux for assistance with experiments, C. Valiquette for his expertise in computer programming and F. Bernard for help with the figures. We also thank E. Hamid and M. Alpert for comments and critical discussion of this manuscript. This work was funded by a grant from the National Institute of Neurological Disorders and Stroke (R01 NS052699) to S.A. and Individual and Group grants from the Canadian Institutes of Health Research (grant numbers 15129 and 15176), the Natural Sciences and Engineering Research Council of Canada (grant number 217435-01) and the Groupe de recherche sur le système nerveux central from the Fonds de la Recherche en Santé du Québec (grant number 5249 to R.D.). L.J. received a Jasper fellowship from the Groupe de recherche sur le système nerveux central.
Author information
Authors and Affiliations
Contributions
R.S. performed experiments and analyses and drafted the paper. L.J. performed experiments on the semi-intact preparation, performed analyses and helped edit the paper. R.D. helped supervise the project and edited and wrote sections of the paper. S.A. supervised the project, performed experiments and analyses, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 2783 kb)
Rights and permissions
About this article
Cite this article
Smetana, R., Juvin, L., Dubuc, R. et al. A parallel cholinergic brainstem pathway for enhancing locomotor drive. Nat Neurosci 13, 731–738 (2010). https://doi.org/10.1038/nn.2548
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2548
This article is cited by
-
Olfactory-induced locomotion in lampreys
Cell and Tissue Research (2022)
-
Parallel descending dopaminergic connectivity of A13 cells to the brainstem locomotor centers
Scientific Reports (2018)
-
A computational model of visually guided locomotion in lamprey
Biological Cybernetics (2013)
-
Cholinergic Receptor Alterations in the Brain Stem of Spinal Cord Injured Rats
Neurochemical Research (2013)
-
A neural system for boosting locomotion
Nature Neuroscience (2010)