Abstract
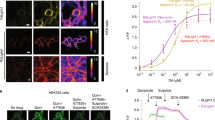
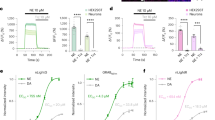
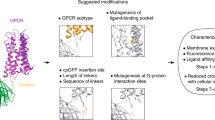
Tools from molecular biology, combined with in vivo optical imaging techniques, provide new mechanisms for noninvasively observing brain processes. Current approaches primarily probe cell-based variables, such as cytosolic calcium or membrane potential, but not cell-to-cell signaling. We devised cell-based neurotransmitter fluorescent engineered reporters (CNiFERs) to address this challenge and monitor in situ neurotransmitter receptor activation. CNiFERs are cultured cells that are engineered to express a chosen metabotropic receptor, use the Gq protein–coupled receptor cascade to transform receptor activity into a rise in cytosolic [Ca2+] and report [Ca2+] with a genetically encoded fluorescent Ca2+ sensor. The initial realization of CNiFERs detected acetylcholine release via activation of M1 muscarinic receptors. We used chronic implantation of M1-CNiFERs in frontal cortex of the adult rat to elucidate the muscarinic action of the atypical neuroleptics clozapine and olanzapine. We found that these drugs potently inhibited in situ muscarinic receptor activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Agnati, L.F. et al. Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol. (Oxf.) 187, 329–344 (2006).
Beaudet, A. & Descarries, L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience 3, 851–860 (1978).
Tsien, R.Y. Building and breeding molecules to spy on cells and tumors. FEBS Lett. 579, 927–932 (2005).
Hires, S.A., Zhu, Y. & Tsien, R.Y. Optical measurement of synaptic glutamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Proc. Natl. Acad. Sci. USA 105, 4411–4416 (2008).
Okumoto, S. et al. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc. Natl. Acad. Sci. USA 102, 8740–8745 (2005).
Vilardaga, J.-P., Bünemann, M., Krasel, C., Castro, M. & Lohse, M.J. Measurement of the millisecond activation switch of G protein−coupled receptors in living cells. Nat. Biotechnol. 21, 807–812 (2003).
Young, S.H. & Poo, M.M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature 305, 634–637 (1983).
Haas, B. et al. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb. Cortex 16, 237–246 (2006).
Schroeder, K.S. & Neagle, B. FLIPR: A new instrument for accurate, high throughput optical screening. J. Biomol. Screen. 1, 75–80 (1995).
Duff Davis, M. & Schmidt, J.J. In vivo spectrometric calcium flux recordings of intrinsic Caudate-Putamen cells and transplanted IMR-32 neuroblastoma cells using miniature fiber optrodes in anesthetized and awake rats and monkeys. J. Neurosci. Methods 99, 9–23 (2000).
Umbriaco, D., Watkins, K.C., Descarries, L., Cozzari, C. & Hartman, B.K. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J. Comp. Neurol. 348, 351–373 (1994).
Everitt, B.J. & Robbins, T.W. Central cholinergic systems and cognition. Annu. Rev. Psychol. 48, 649–684 (1997).
Raedler, T.J. et al. Towards a muscarinic hypothesis of schizophrenia. Mol. Psychiatry 12, 232–246 (2007).
Levey, A.I., Kitt, C.A., Simonds, W.F., Price, D.L. & Brann, M.R. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 11, 3218–3226 (1991).
Mank, M. et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat. Methods 5, 805–811 (2008).
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Rasmusson, D.D., Clow, K. & Szerb, J.C. Frequency-dependent increase in cortical acetylcholine release evoked by stimulation of the nucleus basalis magnocellularis in the rat. Brain Res. 594, 150–154 (1992).
Day, J.C., Kornecook, T.J. & Quirion, R. Application of in vivo microdialysis to the study of cholinergic systems. Methods 23, 21–39 (2001).
Rasmusson, D.D., Clow, K. & Szerb, J.C. Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience 60, 665–677 (1994).
Berg, R.W., Friedman, B., Schroeder, L.F. & Kleinfeld, D. Activation of nucleus basalis facilitates cortical control of a brainstem motor program. J. Neurophysiol. 94, 699–711 (2005).
Meltzer, H.Y. What's atypical about atypical antipsychotic drugs? Curr. Opin. Pharmacol. 4, 53–57 (2004).
Snyder, E.M. & Murphy, M.R. Schizophrenia therapy: beyond atypical antipsychotics. Nat. Rev. Drug Discov. 7, 471–472 (2008).
Bymaster, F.P. et al. Muscarinic mechanisms of antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 1125–1143 (2003).
Nguyen, Q.T., Yang, J. & Miledi, R. Effects of atypical antipsychotics on vertebrate neuromuscular transmission. Neuropharmacology 42, 670–676 (2002).
Parada, M.A. & Hernandez, L. Selective action of acute systemic clozapine on acetylcholine release in the rat prefrontal cortex by reference to the nucleus accumbens and striatum. J. Pharmacol. Exp. Ther. 281, 582–588 (1997).
Ichikawa, J., Dai, J., O'Laughlin, I.A., Fowler, W.L. & Meltzer, H.Y. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacology 26, 325–339 (2002).
Bolden, C., Cusack, B. & Richelson, E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 260, 576–580 (1992).
Chew, M.L. et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr. Res. 88, 63–72 (2006).
Davies, M.A., Compton-Toth, B.A., Hufeisen, S.J., Meltzer, H.Y. & Roth, B.L. The highly efficacious actions of N-desmethylclozapine at muscarinic receptors are unique and not a common property of either typical or atypical antipsychotic drugs: Is M1 agonism a pre-requisite for mimicking clozapine's actions. Psychopharmacology (Berl.) 178, 451–460 (2005).
Johnson, D.E. et al. The role of muscarinic receptor antagonism in antipsychotic-induced hippocampal acetylcholine release. Eur. J. Pharmacol. 506, 209–219 (2005).
Gray, J.A. & Roth, B.L. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr. Bull. 33, 1100–1119 (2007).
Tani, Y., Saito, K., Imoto, M. & Ohno, T. Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain: an in vivo microdialysis study. Eur. J. Pharmacol. 351, 181–188 (1998).
Snyder, S., Greenberg, D. & Yamamura, H.I. Antischizophrenic drugs and brain cholinergic receptors. Affinity for muscarinic sites predicts extrapyramidal effects. Arch. Gen. Psychiatry 31, 58–61 (1974).
Krasel, C., Vilardaga, J.P., Bunemann, M. & Lohse, M.J. Kinetics of G protein–coupled receptor signaling and desensitization. Biochem. Soc. Trans. 32, 1029–1031 (2004).
Vögler, O. et al. Receptor subtype-specific regulation of muscarinic acetylcholine receptor sequestration by dynamin. Distinct sequestration of m2 receptors. J. Biol. Chem. 273, 12155–12160 (1998).
Willars, G.B. & Nahorski, S.R. Quantitative comparisons of muscarinic and bradykinin receptor–mediated Ins (1,4,5)P3 accumulation and Ca2+ signaling in human neuroblastoma cells. Br. J. Pharmacol. 114, 1133–1142 (1995).
Horowitz, L.F. et al. Phospholipase C in living cells: Activation, inhibition, Ca2+ requirement, and regulation of M current. J. Gen. Physiol. 126, 243–262 (2005).
Zhang, J., Hupfeld, C.J., Taylor, S.S., Olefsky, J.M. & Tsien, R.Y. Insulin disrupts beta-adrenergic signaling to protein kinase A in adipocytes. Nature 437, 569–573 (2005).
Kostenis, E., Waelbroeck, M. & Milligan, G. Techniques: promiscuous Gα proteins in basic research and drug discovery. Trends Pharmacol. Sci. 26, 595–602 (2005).
Coward, P., Chan, S.D., Wada, H.G., Humphries, G.M. & Conklin, B.R. Chimeric G proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal. Biochem. 270, 242–248 (1999).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Tsai, P.S. & Kleinfeld, D. In vivo two-photon laser-scanning microscopy with concurrent plasma-mediated ablation: principles and hardware realization. in Methods for In vivo Optical Imaging, 2nd edn (ed. R.D. Frostig) 59–115 (CRC Press, Boca Raton, 2009).
Nguyen, Q.-T., Dolnick, E.M., Driscoll, J. & Kleinfeld, D. MPScope 2.0: A computer system for two-photon laser scanning microscopy with concurrent plasma-mediated ablation and electrophysiology. in Methods for In vivo Optical Imaging 2nd edn (ed. R.D. Frostig) 117–142 (CRC Press, Boca Raton, 2009).
Drobizhev, M., Tillo, S., Makarov, N.S., Hughes, T.E. & Rebane, A. Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J. Phys. Chem. B 113, 855–859 (2009).
Schaffer, C.B. et al. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol. 4, e22 (2006).
Metherate, R. & Ashe, J.H. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J. Neurosci. 12, 5312–5323 (1993).
Kawaja, M.D. & Gage, F.H. Morphological and neurochemical features of cultured primary skin fibroblasts of Fischer 344 rats following striatal implantation. J. Comp. Neurol. 317, 102–116 (1992).
Nishimura, N. et al. Targeted insult to individual subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat. Methods 3, 99–108 (2006).
Shirazi-Southall, S., Rodriguez, D.E. & Nomikos, G.G. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology 26, 583–594 (2002).
DiMatteo, I., Genovese, C.R. & Kass, R.E. Bayesian curve-fitting with free-knot splines. Biometrika 88, 1055–1071 (2001).
Acknowledgements
We are grateful to T. Bartfai, D.K. Berg, J.-P. Changeux, J.M. Edeline, A.L. Fairhall, B. Hille, H.J. Karten, R. Metherate, P.A. Slesinger, T. Talley, R.Y. Tsien and M. Tuszynski for valuable discussions. We thank R. Figueroa for maintaining the cell culture facility, J. Groisman for preparing the artwork in Figure 2a and A. Miyanohara (Vector Development Laboratory, Human Gene Therapy Program, University of California San Diego) for producing the lentiviruses. This work was supported by the US National Institutes of Health Medical Scientist Training Program (L.F.S.), the Max Planck Society (O.G.) and grants from the US National Institutes of Health (DA024206, EB003832 and MH085499 to D.K., GM18360 and DA19372 to P.T., and MH070655 to Q.-T.N.).
Author information
Authors and Affiliations
Contributions
D.K., Q.-T.N. and L.F.S. designed and made the CNiFERs, O.G. and M.M. synthesized the calcium indicator and consulted on molecular biology, D.K., A.M., Q.-T.N., L.F.S. and P.T. characterized and applied the CNiFERS, and D.K., Q.-T.N. and L.F.S. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
David Kleinfeld, Lee F. Schroeder and Quoc-Thang Nguyen are the authors of a patent application related to the paper and are entitled to receive royalties if a patent is granted. FemtoScience, of which Quoc-Thang Nguyen is CEO and founder, has licensed the intellectual property from the University of California San Diego.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 3225 kb)
Rights and permissions
About this article
Cite this article
Nguyen, QT., Schroeder, L., Mank, M. et al. An in vivo biosensor for neurotransmitter release and in situ receptor activity. Nat Neurosci 13, 127–132 (2010). https://doi.org/10.1038/nn.2469
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2469
This article is cited by
-
Human stem cell-based models for studying autism spectrum disorder-related neuronal dysfunction
Molecular Autism (2020)
-
An optimized acetylcholine sensor for monitoring in vivo cholinergic activity
Nature Methods (2020)
-
Designing a norepinephrine optical tracer for imaging individual noradrenergic synapses and their activity in vivo
Nature Communications (2018)
-
A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies
Nature Biotechnology (2018)
-
Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex
Nature Communications (2016)