Abstract
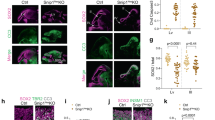
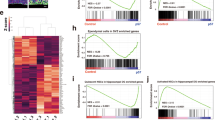
The development of the brain requires the exquisite coordination of progenitor proliferation and differentiation to achieve complex circuit assembly. It has been suggested that glycogen synthase kinase 3 (GSK-3) acts as an integrating molecule for multiple proliferation and differentiation signals because of its essential role in the RTK, Wnt and Shh signaling pathways. We created conditional mutations that deleted both the α and β forms of GSK-3 in mouse neural progenitors. GSK-3 deletion resulted in massive hyperproliferation of neural progenitors along the entire neuraxis. Generation of both intermediate neural progenitors and postmitotic neurons was markedly suppressed. These effects were associated with the dysregulation of β-catenin, Sonic Hedgehog, Notch and fibroblast growth factor signaling. Our results indicate that GSK-3 signaling is an essential mediator of homeostatic controls that regulate neural progenitors during mammalian brain development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Doble, B.W. & Woodgett, J.R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 (2003).
Kockeritz, L., Doble, B., Patel, S. & Woodgett, J.R. Glycogen synthase kinase-3: an overview of an over-achieving protein kinase. Curr. Drug Targets 7, 1377–1388 (2006).
Beaulieu, J.M., Gainetdinov, R.R. & Caron, M.G. The Akt–GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 28, 166–172 (2007).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 (2004).
Ying, Q.L. et al. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 (2008).
Bone, H.K. et al. Involvement of GSK-3 in regulation of murine embryonic stem cell self-renewal revealed by a series of bisindolylmaleimides. Chem. Biol. 16, 15–27 (2009).
Mao, Y. et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136, 1017–1031 (2009).
Corbin, J.G. et al. Regulation of neural progenitor cell development in the nervous system. J. Neurochem. 106, 2272–2287 (2008).
Shimizu, T. et al. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol. Cell. Biol. 28, 7427–7441 (2008).
Knoepfler, P.S. & Kenney, A.M. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle 5, 47–52 (2006).
Bechard, M. & Dalton, S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol. Cell. Biol. 29, 2092–2104 (2009).
Doble, B.W. & Woodgett, J.R. Exploring pluripotency with chemical genetics. Cell Stem Cell 4, 98–100 (2009).
Otto, T. et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 15, 67–78 (2009).
Jia, J. et al. Shaggy/GSK3 antagonizes Hedgehog signaling by regulating Cubitus interruptus. Nature 416, 548–552 (2002).
Espinosa, L., Ingles-Esteve, J., Aguilera, C. & Bigas, A. Phosphorylation by glycogen synthase kinase 3 beta downregulates Notch activity, a link for Notch and Wnt pathways. J. Biol. Chem. 278, 32227–32235 (2003).
Uemura, K. et al. GSK3beta activity modifies the localization and function of presenilin 1. J. Biol. Chem. 282, 15823–15832 (2007).
Jin, Y.H., Kim, H., Oh, M., Ki, H. & Kim, K. Regulation of Notch1/NICD and Hes1 expressions by GSK-3alpha/beta. Mol. Cells 27, 15–19 (2009).
Tronche, F. et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99–103 (1999).
Yokota, Y. et al. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron 61, 42–56 (2009).
MacAulay, K. et al. Glycogen synthase kinase 3alpha–specific regulation of murine hepatic glycogen metabolism. Cell Metab. 6, 329–337 (2007).
Patel, S. et al. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol. Cell. Biol. 28, 6314–6328 (2008).
Kim, W.Y. et al. Essential roles for GSK-3s and GSK-3–primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52, 981–996 (2006).
Sessa, A., Mao, C.A., Hadjantonakis, A.K., Klein, W.H. & Broccoli, V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron 60, 56–69 (2008).
Ding, Q. et al. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development 125, 2533–2543 (1998).
Chenn, A. & Walsh, C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002).
Woodhead, G.J., Mutch, C.A., Olson, E.C. & Chenn, A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J. Neurosci. 26, 12620–12630 (2006).
Chen, S. et al. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor–mediated transcription. J. Cell Biol. 152, 87–96 (2001).
Götz, M. & Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788 (2005).
Dehay, C. & Kennedy, H. Cell-cycle control and cortical development. Nat. Rev. Neurosci. 8, 438–450 (2007).
Zechner, D. et al. Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. 258, 406–418 (2003).
Machon, O. et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev. Biol. 311, 223–237 (2007).
Gulacsi, A.A. & Anderson, S.A. Beta-catenin–mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat. Neurosci. 11, 1383–1391 (2008).
Yoon, K. & Gaiano, N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 8, 709–715 (2005).
Mizutani, K., Yoon, K., Dang, L., Tokunaga, A. & Gaiano, N. Differential Notch signaling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 (2007).
Shimojo, H., Ohtsuka, T. & Kageyama, R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 58, 52–64 (2008).
He, T.C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Calvisi, D.F., Ladu, S., Factor, V.M. & Thorgeirsson, S.S. Activation of beta-catenin provides proliferative and invasive advantages in c-myc/TGF-alpha hepatocarcinogenesis promoted by phenobarbital. Carcinogenesis 25, 901–908 (2004).
Eilers, M. & Eisenman, R.N. Myc's broad reach. Genes Dev. 22, 2755–2766 (2008).
Cappello, S. et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 9, 1099–1107 (2006).
Acknowledgements
We thank F. Polleux, L. Pevny and E. Anton for valuable advice and comments on the manuscript. We are also grateful to L. Goins and A. McKell for animal care and M. Aita for technical support. This research was supported by grants from the US National Institutes of Health (NS050968 to W.D.S. and NS045892, which supports the Confocal and Multiphoton Imaging and Expression Localization Cores of University of North Carolina Neuroscience Center) and Canadian Institutes of Health Research (MOP 74711 to J.R.W.).
Author information
Authors and Affiliations
Contributions
W.-Y.K. designed and conducted most of the experiments, analyzed the data, and co-wrote the paper. X.W. contributed to the in vitro experiments and ideas. Y.W. conducted western blotting and contributed technical assistance. B.W.D. and S.P. generated the GSK-3 mutant lines. J.R.W. contributed to experimental design, provided intellectual guidance and co-wrote the paper. W.D.S. supervised the work, contributed to the experimental design, provided intellectual guidance and co-wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 527 kb)
Rights and permissions
About this article
Cite this article
Kim, WY., Wang, X., Wu, Y. et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci 12, 1390–1397 (2009). https://doi.org/10.1038/nn.2408
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2408
This article is cited by
-
Disrupted extracellular matrix and cell cycle genes in autism-associated Shank3 deficiency are targeted by lithium
Molecular Psychiatry (2023)
-
Light at night: effect on the daily clock, learning, memory, cognition, and expression of transcripts in different brain regions of rat
Photochemical & Photobiological Sciences (2023)
-
GSK3β is a critical, druggable component of the network regulating the active NOTCH1 protein and cell viability in CLL
Cell Death & Disease (2022)
-
WNK1/HSN2 mediates neurite outgrowth and differentiation via a OSR1/GSK3β-LHX8 pathway
Scientific Reports (2022)
-
MACF1, Involved in the 1p34.2p34.3 Microdeletion Syndrome, is Essential in Cortical Progenitor Polarity and Brain Integrity
Cellular and Molecular Neurobiology (2022)